733
Views & Citations10
Likes & Shares
Ascorbic acid is widely known for its role
as an antioxidant and enzyme cofactor and it plays a dynamic role in
maintaining good health. The study was performed on the test samples to
determine the impact of the Consciousness Energy Healing Treatment (Trivedi
Effect®) on the physicochemical and thermal properties of ascorbic
acid using modern analytical techniques. In this experiment, the test sample
was divided into two parts. One part was not given any treatment called the
control sample, while the other part was received the Consciousness Energy Healing
Treatment by a well-known Biofield Energy Healer, Dahryn Trivedi, known as the
treated sample. The PXRD data of the treated sample revealed the significant
alterations in the peak intensities and crystallite sizes ranging from -77.58%
to 368.42% and -42.26% to 163.08%, respectively; however, the average
crystallite size was increased by 5.18% compared with the control sample. The
particle size distribution of the treated sample was increased by 12.67% (d10),
29.60% (d50), 18.29% (d90), and 22.35% {D(4,3)}, which causes 13.95%
significant decrease in the specific surface area as compared to the control
sample. The weight loss of the treated sample was 8.23% decreased, but the
residue weight was significantly increased by 39.79% compared with the control
sample. The melting temperature of the treated sample was slightly increased by
0.42%; while the decomposition temperature was decreased by 7.60%, compared to
the control sample. Besides, the treated sample showed alterations in the
latent heat of fusion and decomposition by 7.63% and -12.96%, respectively
compared with the control sample. The results indicated that the Trivedi Effect®-Consciousness
Energy Healing Treatment might form a novel polymorph of the ascorbic acid that
might ensure better appearance, flowability and content uniformity during the
product formulation along with improved thermal stability as compared to the
untreated sample. Thus, the use of the Biofield Energy Treated ascorbic acid
might be proved beneficial in the formulation of various
nutraceutical/pharmaceutical preparations.
Keywords: Ascorbic acid, The Trivedi Effect®, Consciousness energy
healing treatment, Particle size, PXRD, TGA/DTG, DSC
INTRODUCTION
Appropriate levels of nutrients are essential for proper The oxido-reduction
reactions play a crucial role in the various metabolic and energetic exchanges
taking in between the living organisms and their surrounding environment. The
ascorbate is an important member of such reactions as it plays the role of an
antioxidant and enzyme cofactor in this redox interrelationship. Ascorbic acid
is mainly a reducing agent that acts as an antioxidant in various free
radical-mediated oxidation processes. Besides, it may also increase the
pro-oxidant chemistry of the redox-active metals such as iron and copper, by
reducing them. Hence, ascorbate is known for its antioxidant activity as well
as a pro-oxidant [1,2]. The role of ascorbic acid is also widely known in
various hydroxylation reactions. The processes involve the formations of
neurotransmitters and hormones in which there are various mono- and di-oxygenation
reactions dependant on ascorbate. Also, ascorbic acid is needed in the
hydroxylation of carnitine [3]. Moreover, ascorbic acid plays an essential role
in humans due to its function in the redox homeostasis. There are two main
forms of vitamin C in the diet, which are L-ascorbic acid and its oxidized
form, dehydroascorbic acid (DHA). In humans, the vitamin C requirement could be
fulfilled by
the natural sources
or
The efficacy and bioavailability of a drug depend upon its physicochemical
and various other properties such as the particle size, crystalline properties,
surface area and stability, etc. [10,11]. Therefore, various techniques were
used by scientists to improve the physiochemical and thermal profile of drugs
and thereby their efficacy and bioavailability within the body. One such
approach that is widely known these days for its significant impact on the
properties of a drug is the concept of the Biofield Energy Treatment. It is an
emerging field that is used as an integrated healthcare approach under the
Complementary and Alternative Medicine (CAM) therapies and provides beneficial
effects against various health conditions [12,13]. National Institute of Health
(NIH) also recommend such Energy Healing therapies under CAM category such as
yoga, natural products, deep breathing, homeopathy, meditation, progressive
relaxation, acupressure, hypnotherapy, acupuncture, healing touch, pilates,
Ayurvedic medicine, Reiki, traditional Chinese herbs and medicines, etc. and
these therapies are widely accepted by most of the USA population [14,15]. In a
similar manner, the Biofield Energy Treatment (The Trivedi Effect®)
has also been known worldwide as it poses a significant impact on the living
organisms and non-living materials. The concept behind this treatment is that
all human beings are infused with a subtle form of energy, which is called as
the putative energy fields (Biofield) [16]. The Trivedi Effect®-Consciousness
Energy Healing Treatment has widely affected the physicochemical and thermal
properties of various pharmaceutical/nutraceutical compounds [17-19], altered
the characteristics of microbes in microbiology [20-22], agriculture science
[23,24], livestock [25], metals, ceramics and polymers [26,27], biotechnology
[28] and skin health [29,30]. Thus, the aim of this study was to determine the
effect of the Trivedi Effect® on the physicochemical properties of
ascorbic acid by using various analytical techniques.
METHODS AND MATERIALS
Chemicals and reagents
Ascorbic acid was purchased from Alfa Aesar, USA and the other
chemicals used during the experiments were of analytical standard procured in
India.
Consciousness energy healing treatment
strategies
The test sample used in the experiment, i.e., ascorbic acid was first
divided into two parts. The first part of the sample was not given any
treatment and labelled as the control sample. On the other hand, the second
part of the sample was known as the Biofield Energy Treated sample and received
the Trivedi Effect®-Consciousness Energy Healing Treatment remotely
under standard laboratory conditions for 3 min. This treatment was provided by
the renowned Biofield Energy Healer, Dahryn Trivedi, USA, through the unique
energy transmission process to the test sample. For the comparison, the control
sample was treated by a “sham” healer as the “sham” healer did not have any
knowledge about the Biofield Energy Treatment. Thereafter, both the samples of
the ascorbic acid were kept in sealed conditions and characterized further
using modern analytical techniques for determining the impact of the Biofield
Energy Treatment on the sample in comparison to the untreated sample.
Characterization
The powder X-ray diffraction (PXRD) analysis of ascorbic acid powder
sample was performed with the help of Rigaku MiniFlex-II Desktop X-ray
diffractometer (Japan) [31,32]. The average size of crystallites was calculated
from PXRD data using the Scherrer’s formula (1):
G = kλ/βcosθ (1)
Where G is the crystallite size in nm, k is the equipment constant, λ
is the radiation wavelength, β is the full-width at half maximum and θ is the
Bragg angle [33].
The particle size analysis (PSA) of ascorbic acid powder was performed
using Malvern Mastersizer 2000 (UK) using the wet method [34,35]. Similarly,
the differential scanning calorimetry (DSC) analysis of ascorbic acid was
performed with the help of DSC Q200, TA instruments. The thermogravimetric
analysis (TGA) thermograms of ascorbic acid were obtained with the help of TGA
Q50 TA instruments [36].
All the experiment was performed three times in order to avoid any error during the experiment. The % change in peak intensity, crystallite size, specific surface area, particle size, latent heat, melting point, weight loss and the maximum thermal degradation temperature of the Biofield Energy Treated ascorbic acid was calculated compared with the control ascorbic acid using the following equation 2:
% change = [Treated — Control]/Control * 100 (2)
RESULTS AND DISCUSSION
Powder x-ray diffraction (PXRD) analysis
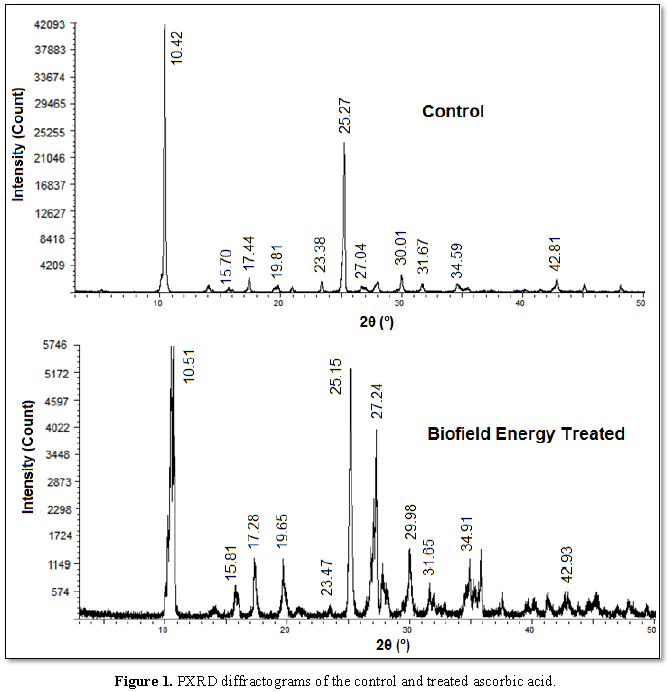
The PXRD study showed the diffractograms of the control and treated
samples (Figure 1) from which the
further analysis was done by comparing the relative intensity and the
crystallite sizes of the characteristic peaks (Table 1) of both the samples. There was the presence of sharp and
intense peaks in the diffractograms of the control and treated samples, which
indicated their crystalline nature. However, there were some alterations in the
Bragg’s angles of the characteristic peaks of the treated sample as compared to
the control sample that denoted the impact of the Biofield Energy Treatment on
the crystalline properties of ascorbic acid.
The analysis of the peak
intensities and crystallite sizes corresponding to the characteristic peaks of
the treated sample showed some significant changes, as the relative intensities
were altered ranging from -77.58% to 368.42%; while the crystallite sizes were
changed ranging from -42.26% to 163.08%, in comparison to the control sample.
The impact of the Biofield Energy Treatment was also visible on the average
crystallite size, as it was increased by 5.18% in the treated sample (570.36
nm) as compared to the control sample (542.27 nm). The previous studies signify
the impact of altered peak intensities and crystallite size on the crystalline
properties of the sample in terms of changes in the crystal morphology that
might indicate the formation of novel polymorph [37,38] of the treated ascorbic
acid. Such techniques of changing the crystal properties and habit could be
used in improving the efficacy and bioavailability of the compound [39]. Hence,
it is presumed that the treated ascorbic acid sample might form a new polymorph
that might show improved efficacy and bioavailability after the Biofield Energy
Treatment compared to the untreated sample.
Particle size analysis (PSA)
The particle size analysis of both the samples helps in analysing the
effect of the Biofield Energy Treatment on the particle size distribution of
the ascorbic acid corresponding at d10, d50, d90 and D(4, 3) in comparison to
the untreated sample (Table 2). The
results indicated the particle size distributions of the treated sample were
significantly increased by 12.67% (d10), 29.60% (d50), 18.29% (d90), and 22.35%
{D(4, 3)} as compared to the control sample.
The resultant specific surface area of the treated sample (0.037 m2/g)
was significantly reduced by 13.95% due to the increase in the particle size,
as compared to the control sample (0.043 m2/g). Various scientific
studies specified the effect of the particle size distribution of the
formulation criteria of the drug such as its compactibility, blend uniformity
and flowability, etc. Such properties further affect the efficacy, safety,
stability, and quality control of the nutraceutical/pharmaceutical formulation
[40,41]. The treated ascorbic acid sample might show better flowability,
content uniformity, and compactibility after the Biofield Energy Treatment in
comparison to the control sample.
Thermal gravimetric analysis
(TGA)/differential thermogravimetric analysis (DTG)
The impact of heat on the stability profile of the control and treated
ascorbic acid samples were analysed along with its degradation pattern by the
TGA/DTG technique. The scientific literature reported the stability of ascorbic
acid till ~200°C when heated and afterward the degradation started leaving the
non-decomposed and carbonaceous residues of ascorbic acid in the form of
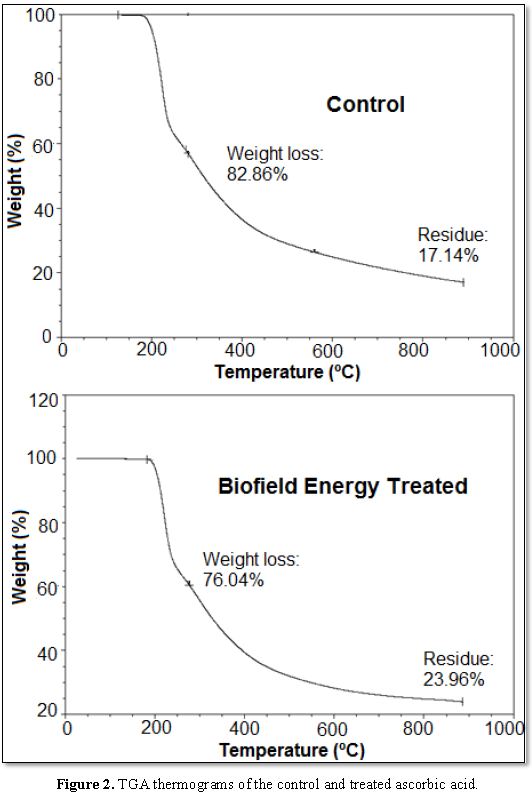
residual mass [42]. The TGA analysis data of the control and treated samples (Figure 2) also indicated the stability
of samples till 200°C as reported in scientific literature. The total weight
loss of the treated ascorbic acid sample was decreased significantly by 8.23%
during the thermal heating, as it was observed to be 76.04% as compared to the
control sample (82.86%). The significant reduction in weight loss of the
treated sample contributed to the remarkable increase in the residue amount by
39.79% after the thermal degradation (Table 3) in comparison to the control sample’s residue. Thus, it showed the
increased thermal stability and reduced degradation of the treated sample
compared to the control sample.
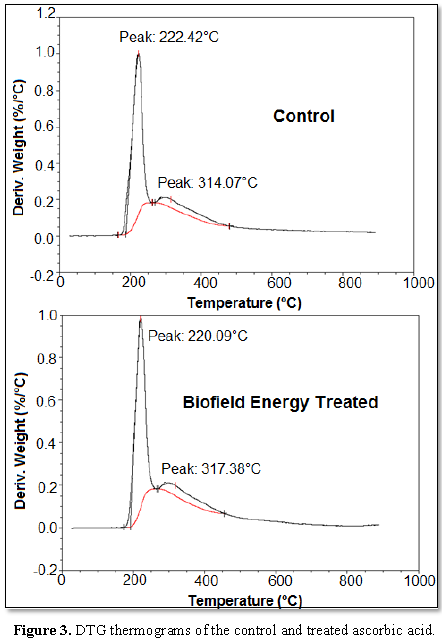
Moreover, the DTG data indicated the two peaks in the thermograms of
both the samples (Figure 3) that
denoted the temperature (Tmax) at which maximum thermal degradation
has taken place. The treated sample’s thermogram showed Tmax at 220.09°C and
317.38°C for the 1st and 2nd peak, respectively in
comparison to the Tmax of the control sample that was observed at
222.42°C and 314.07°C, respectively. Thus, the treated sample showed 1.05%
decrease in the Tmax of the 1st peak, while the 2nd
peak was increased by 1.05% as compared to the control sample. Hence, the study
indicated the increased thermal stability of the treated sample at higher
temperature range as compared to the control ascorbic acid sample. The overall
TGA/DTG studies revealed the significant reduction in the thermal degradation
of the treated sample that indicated the increased thermal stability of the
ascorbic acid after the Biofield Energy Treatment.
Differential scanning calorimetry (DSC)
analysis
The DSC analysis helps in analysing the difference between the control
and treated ascorbic acid sample in terms of their melting and decomposition
temperatures along with the latent heat used during the process in the process
of heating [43]. The scientific studies reported the presence of an endothermic
peak (melting peak) at 193°C in the DSC thermogram of ascorbic acid when heated
at the rate of 10°C/min. Moreover, an exothermic peak was also reported in the
thermogram that is considered as the thermal decomposition of the ascorbic acid
during its further heating that resulted in the release of the volatile
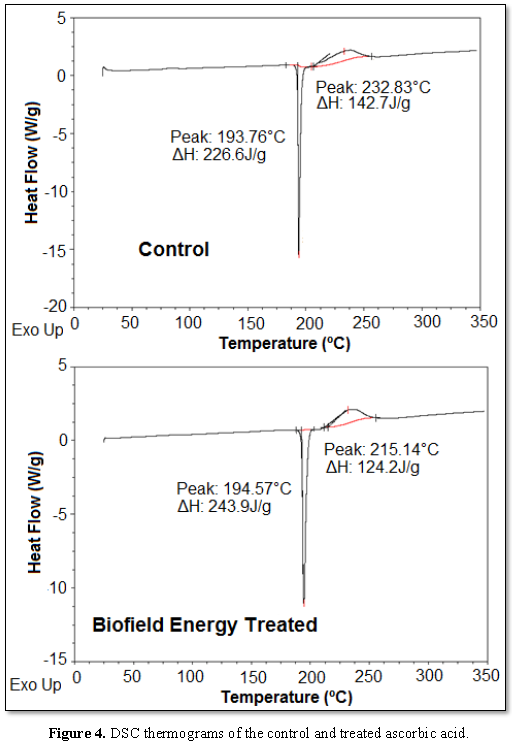
compounds thereby forming the carbonaceous residue [42]. The DSC thermograms of
the control and the treated samples (Figure
4) were observed similarly as mentioned in the previous studies. The
further analysis reported that the treated sample showed the endothermic peak
with minor increase in the melting temperature (0.42%), while the ΔHfusion
was increased by 7.63% (Table 4) as compared to the control sample. Besides, the exothermic peak
(decomposition temperature) of the treated sample was present at 215.14°C,
which was decreased by 7.60% (~17°C) in comparison to the degradation
temperature of the control sample. Also, the treated sample showed a reduction
in the ΔHdecomposition by 12.96%, compared to the control ascorbic
acid sample (Table 4).
Hence, the overall DSC data revealed the significant decrease in the
decomposition temperature along with remarkable alterations in ΔHfusion
and ΔHdecomposition of the treated sample after the Biofield Energy
Treatment that might indicate some changes in the crystallization structure and
molecular bonding [43] of the ascorbic acid in comparison to the control
sample.
CONCLUSION
The study concluded the outcome of the Trivedi Effect®-Consciousness
Energy Healing Treatment on the crystalline, physical and thermal properties of
ascorbic acid in comparison to the untreated sample. The PXRD peak intensities
of the treated sample and the corresponding crystallite sizes showed changes
ranging from -77.58% to 368.42% and -42.26% to 163.08%, respectively as
compared to the control sample. Besides, the treated sample showed an increase
in the average crystallite size by 5.18% compared with the control ascorbic
acid sample. Such significant changes in the treated sample might be attributed
to the polymorphic transition of the ascorbic acid sample that may take place
after the Biofield Energy Treatment in comparison to the untreated ascorbic
acid sample. The new polymorph of the treated ascorbic acid might show better
bioavailability and efficacy compared to the control sample. The particle size
data indicated the significant changes in the particle size distribution of the
treated sample corresponding to d10, d50, d90, and D(4,3) that were observed to
be increased by 12.67%, 29.60%, 18.29% and 22.35%, respectively, compared to
the control sample. The significant increase in the particle sizes of the
treated ascorbic acid sample contributed to the decrease in the specific
surface area by 13.95% compared with the control sample. Such alterations in
the particle sizes of the treated sample might improve the appearance,
flowability and content uniformity of ascorbic acid during the formulation
development as compared to the untreated sample. The weight loss of the treated
sample was 8.23% decreased, but the residue weight was significantly increased
by 39.79% compared with the control sample. The melting temperature of the
treated sample was slightly increased by 0.42%; while the decomposition
temperature was decreased by 7.60%, compared to the control sample. Besides,
the treated sample showed alterations in the ΔHfusion and ΔHdecomposition
by 7.63% and -12.96%, respectively compared with the control sample. Hence, the
thermal data of both the samples indicated that the thermal stability of the treated
sample was increased, compared to the untreated ascorbic acid sample. Hence,
the overall results on the Biofield Energy Treated ascorbic acid revealed the
impact of the Trivedi Effect®-Consciousness Energy Healing Treatment
on the physicochemical and thermal properties involving the crystalline
properties, particle sizes, surface area, thermal degradation, and melting
profile, etc. The Biofield Energy Treatment of the sample might form a new
polymorph of the ascorbic acid that may show better appearance, flowability,
content uniformity and efficacy along with improved thermal stability compared
to the untreated sample. Thus, it could be concluded that the Biofield Energy
Treated ascorbic acid could be used in the nutraceutical/pharmaceutical formulation
for providing better prevention and treatment against various diseases such as
scurvy, tuberculosis, common cold, febrile states, hypercholesterolemia,
pneumonia infection, coronary heart disease, whooping cough, rheumatic fever,
hypertension, angina pectoris, congestive cardiac failure, diphtheria, vascular
disorders, diabetes mellitus and sinusitis, glaucoma, autoimmune diseases,
bleeding gums, neurotic disturbances, and may improve the fracture, burns and
wound healing, etc.
ACKNOWLEDGEMENT
The authors are grateful to Central Leather Research Institute, SIPRA
Lab. Ltd., Trivedi Science, Trivedi Global, Inc., Trivedi Testimonials, and
Trivedi Master Wellness for their assistance and support during this work.
1.
Buettner GR, Jurkiewicz BA
(1996) Catalytic metals, ascorbate and free radicals: Combinations to avoid.
Radiat Res 145: 532-541.
2.
Lykkesfeldt J, Michels AJ,
Frei B (2014) Vitamin C. Adv Nutr 5: 16-18.
3.
Englard S, Seifter S (1986)
The biochemical functions of ascorbic acid. Annu Rev Nutr 6: 365-406.
4.
Malo C, Wilson JX (2000)
Glucose modulates vitamin C transport in adult human small intestinal brush
border membrane vesicles. J Nutr 130: 63-69.
5.
Heitzer T, Schlinzig T,
Krohn K, Meinertz T, Münzel T (2001) Endothelial dysfunction, oxidative stress,
and risk of cardiovascular events in patients with coronary artery disease.
Circulation 104: 2673-2678.
6.
Evans JR, Henshaw K (2008)
Antioxidant vitamin and mineral supplements for preventing age-related macular
degeneration. Cochrane Database Syst Rev CD000253.
7.
Mandl J, Szarka A, Bánhegyi
G (2009) Vitamin C: update on physiology and pharmacology. Br J Pharmacol 157:
1097-1110.
8.
Rowan MP, Cancio LC, Elster
EA, Burmeister DM, Rose LF, et al. (2015) Burn wound healing and treatment:
review and advancements. Crit Care 19: 243.
9.
Figueroa-Méndez R,
Rivas-Arancibia S (2015) Vitamin C in health and disease: Its role in the
metabolism of cells and redox state in the brain. Front Physiol 6: 397.
10.
Miranda A, Caraballo I,
Millán M (2002) Stability study of flutamide in solid state and in aqueous
solution. Drug Dev Ind Pharm 28: 413-422.
11.
Anjum S, Swan SK, Lambrecht
LJ, Radwanski E, Cutler DL, et al. (1999) Pharmacokinetics of flutamide in
patients with renal insufficiency. Br J Clin Pharmacol 47: 43-47.
12.
Frass M, Strassl RP, Friehs
H, Müllner M, Kundi M, et al. (2012) Use and acceptance of complementary and
alternative medicine among the general population and medical personnel: A
systematic review. Ochsner J 12: 45-56.
13.
Barnes PM, Bloom B, Nahin
RL (2007) Complementary and alternative medicine use among adults and children:
United States, 2007. Natl Health Stat Report 12: 1-23.
14.
Rubik B (2002) The biofield
hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med
8: 703-717.
15.
Koithan M (2009)
Introducing complementary and alternative therapies. J Nurse Pract 5: 18-20.
16.
Berman JD, Straus SE (2004)
Implementing a research agenda for complementary and alternative medicine. Annu
Rev Med 55: 239-254.
17.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Nykvist CD, et al. (2017) Evaluation of the Trivedi Effect®
- Energy of consciousness energy healing treatment on the physical, spectral
and thermal properties of zinc chloride. Am J Life Sci 5: 11-20.
18.
Trivedi MK, Patil S,
Shettigar H, Bairwa K, Jana S (2015) Spectroscopic characterization of biofield
treated metronidazole and tinidazole. Med Chem 5: 340-344.
19.
Trivedi MK, Branton A,
Trivedi D, Shettigar H, Bairwa K, et al. (2015) Fourier transform infrared and
ultraviolet-visible spectroscopic characterization of biofield treated
salicylic acid and sparfloxacin. Nat Prod Chem Res 3: 186.
20.
Trivedi MK, Patil S,
Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral
load of Hepatitis B and C viruses. J Antivir Antiretrovir 7: 83-88.
21.
Trivedi MK, Patil S,
Shettigar H, Mondal SC, Jana S (2015) An impact of biofield treatment: Anti-mycobacterial
susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis 5: 189.
22.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after
biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin
Med Genom 3: 129.
23.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield
and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica
L.). J Food Nutr Sci 3: 245-250.
24.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of biochemical marker- Glutathione
and DNA fingerprinting of biofield energy treated Oryza sativa. Am J
BioSci 3: 243-248.
25.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Mondal SC, et al. (2015) Effect of biofield treated energized water on
the growth and health status in chicken (Gallus gallusdomesticus). Poult
Fish WildlSci 3: 140.
26.
Trivedi MK, Tallapragada
RM, Branton A, Trivedi D, Nayak G, et al. (2015) The potential impact of biofield energy
treatment on the physical and thermal properties of silver oxide powder. Int J
Biomed Sci Eng 3: 62-68.
27.
Trivedi MK, Tallapragada
RM, Branton A, Trivedi D, Nayak G, et al. (2015) Analysis of physical, thermal, and structural
properties of biofield energy treated molybdenum dioxide. Int J Mater Sci Appl
4: 354-359.
28.
Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ
Health Sci 1: 1-9.
29.
Kinney JP, Trivedi MK,
Branton A, Trivedi D, Nayak G, et al. (2017) Overall skin health potential of the biofield
energy healing based herbomineral formulation using various skin parameters. Am
J Life Sci 5: 65-74.
30.
Smith DM, Trivedi MK,
Branton A, Trivedi D, Nayak G, et al. (2017) Skin protective activity of consciousness
energy healing treatment based herbomineral formulation. J Food Nutr Sci 5: 86-95.
31.
(1997) Desktop X-ray Diffractometer “MiniFlex+”.
Rigaku J 14: 29-36.
32.
Zhang T, Paluch K,
Scalabrino G, Frankish N, Healy AM, et al. (2015) Molecular structure studies of
(1S,2S)-2-benzyl-2,3-dihydro-2-(1Hinden-2-yl)-1H-inden-1-ol. J Mol Struct 1083:
286-299.
33.
Langford JI, Wilson AJC (1978) Scherrer after sixty years: A survey and some new results in the
determination of crystallite size. J Appl Cryst 11: 102-113.
34.
Trivedi MK, Sethi KK, Panda
P, Jana S (2017) Physicochemical, thermal and spectroscopic characterization of sodium
selenate using XRD, PSD, DSC, TGA/DTG, UV-vis and FT-IR. Marmara Pharm J 21/2:
311-318.
35.
Trivedi MK, Sethi KK, Panda
P, Jana S (2017) A comprehensive physicochemical, thermal and
spectroscopic characterization of zinc (II) chloride using X‑ray diffraction,
particle size distribution, differential scanning calorimetry,
thermogravimetric analysis/differential thermogravimetric analysis,
ultraviolet‑visible and Fourier transform‑infrared spectroscopy. Int J Pharm
Investig 7: 33-40.
36.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Plikerd WD, et al. (2017) A systematic study of the biofield energy
healing treatment on physicochemical, thermal, structural and behavioral
properties of iron sulphate. Int J Bioorg Chem 2: 135-145.
37.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Lee AC, et al. (2017) Evaluation of the impact of biofield energy
healing treatment (The Trivedi Effect®) on the physicochemical,
thermal, structural and behavioural properties of magnesium gluconate. Int J
Nutr Food Sci 6: 71-82.
38.
Trivedi MK, Branton A,
Trivedi D, Nayak G, Plikerd WD, et al. (2017) Evaluation of the physicochemical, spectral, thermal and behavioral
properties of sodium selenate: Influence of the energy of consciousness healing
treatment. Am J Quantum Chem Mol Spectroscopy 2: 18-27.
39.
Savjani KT, Gajjar AK,
Savjani JK (2012) Drug solubility: Importance and enhancement
techniques. ISRN Pharmaceutics, Article ID 195727.
40.
Morin G, Briens L (2013) The effect of lubricants on powder flowability for pharmaceutical
application. AAPS Pharm Sci Tech 14: 1158-1168.
41.
Hlinak AJ, Kuriyan K,
Morris KR, Reklaitis GW, et al. (2006) Understanding critical material properties
for solid dosage form design. J Pharm Innov 1: 12-17.
42.
Nunes JFL, Melo DMA, de
Moura MFV, de Farias RF (2007) TG-DSC study of ascorbic acid pharmaceutical
formulations: Sodium croscarmellose, microcrystalline cellulose and lactose as
excipients. Revista Química no Brasil 1: 7-14.
43.
Zhao Z, Xie M, Li Y, Chen
A, Li G, et al. (2015) Formation of curcumin nanoparticles via solution
enhanced dispersion by supercritical CO2. Int J Nanomed 10:
3171-3181.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Astronomy and Space Research