742
Views & Citations10
Likes & Shares
Background: Antiretroviral therapy (ART) enables people infected with the Human
immunodeficiency type 1 virus (HIV) to control viral replication and maintain
undetectable plasma levels of the virus. However elite controllers are a unique
subpopulation of HIV infected people capable of durable natural suppression of
HIV without ART. Although elite controllers (ECS) are extremely rare they hold
great potential in increasing our understanding about immune mechanisms and
correlates of protection necessary for an efficient vaccine or functional cure
of HIV. Cameroon has some of the most diversified HIV strains globally but no
ECS have been described within this region. We present here four Cameroonian
female HIV controllers from the CIRCB AFRODEC cohort of CRFO.2AG infected people.
Methods: We monitored four
members of the CIRCB AFRODEC cohort who maintained persistent suppression of
viral load (HIV RNA <50 copies/ml) during eight years without ART. A
comparative analysis of plasma levels of five inflammatory
Results: Elite controller one
(EC1) was diagnosed at 29 year old in 2003 after the husband died of HIV
infection. Elite controller two (EC2) was diagnosed at 23 years old in 1995
while elite controller three (EC3) and four (EC4) were both diagnosed at 28
years in 2008 respectively. In contrast to the other three elite controllers
EC2 had elevated plasma levels of IP-10 and MIG which was associated with
decreasing helper CD4+ counts. ECI had at least three-fold more plasma levels
of RANTES than the other elite controllers and a durable CD4/CD8 ratio higher
than 2 throughout the study. Low plasma levels of CXCL10/IP-10, MCP-1,
MIG/CXCL9 and IL-8 were associated with elite control of HIV-1 infection. This
is in contrast with RANTES/CCL5 where higher plasma levels correlated with the
best elite controller profile (ECI).
Conclusion: The inflammatory chemokine profiles of the elite controllers were
heterogeneous probably reflecting underlying differences which can ultimately
impact disease progression. This report highlights the need to monitor
inflammatory chemokines and other plasma biomarkers in elite controllers as
additional strategies toward predicting clinical outcomes in the long term
management of elite controllers.
Keywords: Elite controllers, HIV, Inflammatory chemokines, Antiretroviral therapy,
Viremia suppression
Abbreviations: ART: Antiretroviral Therapy; Abs.:
Absolute; EC: Elite Controller; ECS: Elite Controllers; HIV: Human
Immunodeficiency Virus Type 1; MCP-1: Monocyte Chemotactic Protein-1; IFN-γ: Interferon-Gamma;
MIG: Monokine Induced by Gamma Interferon; CCR5: C-C Chemokine Receptor Type 5;
CIRCB: Chantal Biya International Reference Center for Research on the
Prevention and Management of HIV/AIDS; AFRODEC: African Dendritic Cell Targeted
Vaccine Cohort; IP-10: IFN-γ-Inducible Protein 10; RANTES/CCL5: Regulated upon
Activation Normal T-Cell Expressed and Secreted
BACKGROUND
Successful ART
permits infected people to control HIV replication to an undetectable level and
maintain normal helper CD4 T cells counts (>500 cells/ mm3).
Following the advent of ‘test and treat’ an increasing number of HIV infected
people are entering ART [1-3]. However a unique minute fraction of HIV infected
individuals referred to elite controllers is capable of spontaneous control of
HIV without ART [4]. Due to their ability to resist HIV mediated disease
progression and maintain persistent HIV viremia suppression current treatment guidelines
do not clarify if ECS should be given ART [5]. As these individuals maintain
ART independent undetectable viral load (plasma HIV RNA <50 copies/ml) there
is confusion on initiating ART for this subset the reason being that the
ultimate outcome of treatment would be undetectable viral load (plasma HIV RNA
<50 copies/ml) and normal helper CD4 T cell values which they naturally
achieve without ART.
In this regard
persistent ECS are proposed both as a model of functional cure and a potential
source of correlates of immune protection for the development of a vaccine
against HIV [5,6]. In regions of intense concurrent infectious diseases like
sub Saharan Africa little is known about long term ART naïve suppression of HIV
replication. Several factors including genetic [7], immunological [8] and
virological [9] have been associated with elite control of HIV. Prominent
amongst these factors are host immune responses which are necessary in
controlling HIV replication [10-12]. In addition host immune responses in
suppressing viral replication might also dampen the persistent inflammation
associated with HIV infection. Thus measuring systemic biomarkers of
inflammation such as chemokines in ECS could identify factors relevant to elite
control or disease progression which could be used in the long term management
of HIV in ECS.
However ECS is a
heterogeneous population as a result of variations in several characteristics
related to disease progression [13,14]. Well over 28% of ECS for reasons
unknown are reported to lose virological control over time and eventually
progressing in disease [15]. The mechanisms causing accelerated disease
progression in some ECS is not known. Nevertheless, generalized inflammation
and trafficking of activated immune cells to sites of infection are known to
exacerbate disease progression. HIV immune activation induces expression of
inflammatory chemokines IP-10, MCP-1, MIG and ITAC which direct cellular immune
responses to sites of infection [16]. Enhanced expression of chemokine receptors
on lymphocytes (e.g. CXCR3 [17] increases the transit of immune cells to sites
of infection.
To investigate the
impact of inflammatory chemokines on elite control of HIV we measured plasma
levels of IP-10, MIG, MCP-1, RANTES/CCL5 and IL-8 in 16 control HIV negative
participants and four ECS from the CIRCB AFRODEC cohort. Our hypothesis is that
lower levels of plasma inflammatory chemokine could be relevant in persistent
EC of HIV infection. Thus, describing distinct inflammatory chemokines profiles
in relation to EC of HIV can determine factors that would be necessary for
predicting ECS and optimizing relevant biomarkers which can be used in the long
term management of HIV in general. Understanding these factors should also
provide vital information for the design of future vaccines or the pursuit of a
functional cure of HIV.
METHODS
Study population
All participants of
this study were adult participants of the CIRCB AFRODEC cohort [35-37].
Participants were 21 to 65 years old and samples were collected as part of the
CIRCB AFRODEC (African HIV-1 dendritic cell targeted vaccine) study. During the
course of eight years 766 members of CIRCB AFRODEC cohort were monitored in
CIRCB CIRCB (Ethics Protocol numbers: CIRCB/14-11DROS631-1112 and
2014/10/499/CE/CNERSH/SP). In addition to people who did not provide consent,
participants who had been diagnosed with Hepatitis B virus, Hepatitis C virus,
Dengue virus, Mycobacterium tuberculosis, or malaria were excluded from the
study. Absolute numbers of helper CD4+ T-cells for HIV-1 positive participants
were determined in fresh whole blood by BD multi-test CD3/CD8/CD45/CD4 and
TruCount tubes (BD Biosciences, San Jose, USA) according to the manufacturer’s
instructions. Plasma HIV-1 viral load was determined on the m2000rt machine
using the Abbott Real-Time HIV-1 Assay protocol.
Plasma sample collection and processing
About 4 ml of blood
was collected into plastic Vacuum blood spray-coated K2EDTA tubes called
Vacutest (Vacutestkirma, Italy). Subsequently, samples were transported to the
Vaccinology laboratory of Chantal BIYA International Reference Centre (CIRCB)
for storage and analysis. All samples were stored at room temperature and
processed within 4 hours of collection. To obtain plasma, samples were
centrifuged at 2,000 rpm for 10 min at 4°C. The plasma fraction was harvested
sterile under the hood, aliquoted in small single-use volumes and stored at
-20°C until use.
Chemokine plasma levels assays
Measurements of
IP-10/IP-10/CXCL10, MIG, MCP-1/CCL2, RANTES/CCL5 and IL-8 in the plasma samples
were conducted using cytometric bead assay (CBA) (BD Biosciences, USA) according
to manufacturer’s instructions. The data were collected using a BD Canto II
flow cytometer (BD Biosciences, USA) and the results were analyzed in FCAP
Array software (Soft Flow).
HIV infection and CD4 T-cell enumeration
Confirmation of HIV
status was done as described for the CIRCB AFRODEC cohort using the Cameroon’s
national algorithm for the diagnosis of HIV infection as previously reported
for the CIRCB AFRODEC cohort [35-37].
RESULTS
Clinical findings
We followed a
cohort of 766 HIV infected people, median age 32 years at enrollment in CIRCB
from 2011 to 2018. Twenty one participants (2.7%) were identified as viremic
controllers and 4 (0.52%) as ECS (EC1, EC2, EC3 and EC4). ECS members of CIRCB
AFRODEC have a median antiretroviral therapy (ART) naïve period of 14 years.
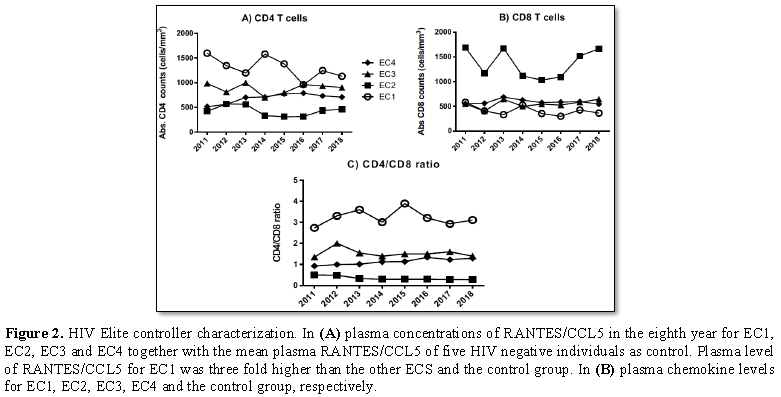
EC1 is a 44 year old
woman who was first diagnosed at the age of 29 years old after the husband who
had already been on ART died of HIV infection in 2003. She was confirmed as
being infected with group M virus by central Pasteur of Cameroon. The viral
load after diagnosis was undetectable and has remained suppressed till the date
of this report. The helper CD4+ T cell count at diagnosis in 2003 was 1445
cells/mm3 with CD4:CD8 ratio of 3.2 and has remained above 900
cells/mm3 throughout the follow up (Figures 1A-1C).
EC2 is a 46 years
old woman who was first diagnosed at the age of 23 years old in 1995 also with
a group M virus. She is a mother of six children all HIV negative and born when
she was already infected with the virus. The husband has remained seronegative
since 1995. In spite the fact that her helper CD4+ T cells count remained below
500 cells/mm3 (Figures 1A-1C) she maintained undetectable
viral load till 2017. Sudden detectable plasma virus was observed in 2017 with
peak viral load (VL) of 3185 RNA copies/ml in 2017 and remained detectable in
2018 (peak VL 11207 copies/ml) when she opted to enter ART.
EC3 is a 37 year
old woman who was first diagnosed at the age of 28 years in 2008. She had a
child in 2010 whom together with the father are HIV negative. Her helper CD4 T
cell count at diagnosis was 984 cells/mm3 with CD4:CD8 ratio of
1.35. Viral load has remained undetectable till date with no dramatic changes
in helper CD4+ T cell counts.
EC4 is also a 37
years old woman first diagnosed at the age of 28 years in 2008. Her helper CD4+
T cell count at diagnosis was 516 cells/mm3 with CD4:Cd8 ratio of
0.93 but has steadily increased over the years to 774 cells/mm3 and
CD4:CD8 ratio of 1.34. VL has remained undetectable throughout the follow up
period.
DISCUSSION
During the last
eight years we identified and monitored four Cameroonian women from the CIRCB
AFRODEC cohort with sustained spontaneous ART independent control of HIV. From
2010 to 2018 all ECS had at least one viral load estimation performed every
year and except for EC2 viral load in all elite controllers (EC1, EC3 and EC4)
remained undetectable without ART for the duration of follow up. Even though
these ECS were enrolled in the CIRCB AFODEC cohort just eight years ago their
cumulative clinical history shows that they have been able to maintain
persistent ART independent suppression of HIV for at least 10 years. In
addition findings from this study like previous reports demonstrate that our
ECS were actually a heterogeneous population [20-22]. Following the advent of
‘test and treat’ in Sub Saharan Africa an increasing number of HIV infected
people are entering ART. However there are no clear cut guidelines on
initiating and evaluating ART effectiveness in ECS. Just about every HIV clade
circulates in Cameroon, so these ECS could potentially yield important and
unique insights into HIV control in the absence of treatment. This makes it
necessary to pursue a continuous assessment of this unique minute fraction of
HIV infected people for the identification of predictive biomarkers [5,6,14,23]
which could become essential in the long term management of the infection in
ECS.
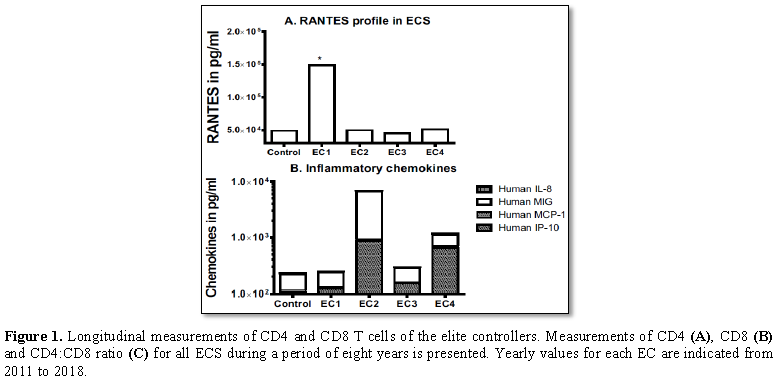
Amongst the 4 ECS
described in this study only one individual (EC2) demonstrated loss of elite
control capability approximately 21 years after the initial diagnosis. This is
similar with previous reports where disease progression in ECS has been
described [14,24-28]. Interestingly EC2 neither transmitted the virus to her
six HIV negative children born after haven been tested HIV positive nor to her
husband who remained negative in spite of repeated exposure. The loss of elite
control capability in EC2 was accompanied by a significant increase in both MIG
and IP-10 (p=0.05) relative to the other ECS. IP-10 and MIG are inflammatory
chemokines which are known to bind on CXCR3 on TH1 cells thereby mobilizing leukocytes
to inflammatory sites. Both chemokines have previously been associated with HIV
mediated immune activation and disease progression [29,30]. The ultimate
emergence of detectable plasma HIV RNA levels during the last two years in EC2
was preceded by persistent progressive helper CD4+ T-cell loss over several
years. This loss of natural suppression of HIV has been reported to occur in
well over 28% of ECS [13,15,28].
In contrast EC1
maintained plasma levels of several inflammatory chemokines including CXCL10/IP-10,
MCP-1, MIG/CXCL9 and Il-8 at values comparable with HIV-negative individuals.
In addition she also showed higher (normal) helper CD4+ T-cells counts and a
CD4:CD8 ratios greater than 2.7 throughout the follow up period. Such low
levels of MIG and IP-10 have also previously been associated with elite control
of HIV [31]. In addition EC1 plasma levels of RANTES/CCL5 were also
comparatively higher (threefold) than for the other ECS and the HIV negative
controls. RANTES/CCL5 is a ligand for CCR5 a major co-receptor for HIV which is
capable of blocking HIV infection. High levels of RANTES in plasma have been
demonstrated to be protective against HIV infection and disease progression
[32,33]. This implies that a combination of several factors would probably be
necessary to defined durable elite control of HIV. On the other hand some
biomarkers such as unusual high plasma levels of IP-10 and MIG in conjunction
with a progressive decline in CD4 T cell levels could constitute early warning
indicators of loss of elite control capability.
CONCLUSION
In conclusion we
have shown that a differential expression of some inflammatory chemokines
including IP-10, MIG and RANTES could be relevant in predicting the long term
outcome of elite control of HIV infection. All ECS had higher levels of IP-10
when compared to HIV negative individuals. Elevated plasma IP-10 levels during
HIV infection have been suggested to be predictive of earlier decline in the
helper CD4 T cell count [34]. In this regard elevated plasma IP-10 and MIG
levels in association with low levels of RANTES/CCL5 might be considered as
predictors of poor outcomes in ECS. On the other hand high levels of
RANTES/CCL5 together with sustained high CD4 T cell counts and a CD4:CD8 ratio
above 2.7 was associated with better outcomes in ECS. Our findings are useful
because these biomarkers could potentially be useful in the long term
management of elite control of HIV infection in sub Saharan Africa.
DECLARATIONS
Ethics approval and consent to participate
This study received
ethical approval from the Cameroon National Ethics Committee for Human Health
Research (Reference numbers CIRCB/14-11/DROS631-1112 and
2014/10/499/CE/CNERSH/SP) and the CIRCB institutional review board (protocol
number 14-11). All participants provided written informed consent. Data were
processed using specific identifiers for privacy and confidentiality purposes.
Clinical data generated during the course of this study was provided free of
charge to all participants.
Consent for publication
EC1, EC2, EC3 and
EC4 provided written informed consent for the publication of this manuscript.
Availability of data and materials
Information
regarding data referring to the CIRCB AFRODEC cohort used in this manuscript is
part of the data bank of the Chantal Biya international Reference center for
research on the prevention and management of HIV/AIDS; it is not possible to
obtain them by URL. All data are fully available without restriction. Data are
available from the CIRCB Institutional Data Access/Ethics Committee for
researchers who meet the criteria for access to confidential data. All requests
for Data should be addressed to the director General of CIRCB reachable by the
following address:
Prof. Alexis Ndjolo,
Director General CIRCB, BP 3077, Messa Yaounde, Cameroon, Tel: +237222315450; Fax:
+237222315456; E-mail: andjolo@yahoo.com; andjolo@circb-cm
Never the less all
supporting datasets relevant to the conclusions of this case report have been
included within the article (five figures).
Competing interests
The authors declare
that they have no competing interests.
Funding
This project was
funded by grants from CIRCB, EDCTP (grant #TA.2010.40200.016)
TWAS(#12059RG/bio/af/ac_G) and Canada grand challenge (#0121-01); to Godwin W
Nchinda; from Korea-Africa cooperation grant (NRF-2013K1A3A1A09076155) from the
National Research Foundation of Korea funded by the Ministry of Science, ICT
and Future Planning in the Republic of Korea to Chae Gyu Park.
Author’s contributions
Conceived and designed the experiments: GWN, LNN
and NNN.
Performed the experiments: LNN, NNN,
AAN, GA, JLS, MSS, SM, ASO, RSK, GOC, ABW, AG, RG.
Technical assistance: RG, AG,
CGP, SM, GOC, MIO, ASO, LK, COE, ABW.
Analyzed the data: GWN,
LNN, NNN and AAA.
Wrote the paper: LNN, GWN and ABW.
The final manuscript was read and approved by all Authors.
Acknowledgement
We would like to
thank the personnel of ‘Unites techniques’ of CIRCB, Centre de Santé Catholique
de Bikop and Mvogbesi Yaounde for their help in collecting the blood samples.
Most importantly our gratitude goes to members of the CIRCB AFRODEC cohort for
consenting to participate in this study. Finally we thank the AFRODEC study
team.
1.
Cohen MS, Chen YQ, McCauley M, Gamble T,
Hosseinipour MC, et al. (2011) Prevention of HIV-1 infection with early
antiretroviral therapy. N Engl J Med 365: 493-505.
2.
Williams BG, Granich R, Dye C (2011) Role of acute
infection in HIV transmission. Lancet 378: 1913; 1914-1915.
3.
Thompson MA, Mugavero MJ, Amico KR, Cargill VA,
Chang LW, et al. (2012) Guidelines for improving entry into and retention in
care and antiretroviral adherence for persons with HIV: Evidence-based
recommendations from an International Association of Physicians in AIDS Care
panel. Ann Intern Med 156: 817-833.
4.
Theze J, Chakrabarti LA, Vingert B, Porichis F,
Kaufmann DE (2011) HIV controllers: A multifactorial phenotype of spontaneous
viral suppression. Clin Immunol 141: 15-30.
5.
Tarancon-Diez L, Dominguez-Molina B, Lopez-Cortes
LF, Ruiz-Mateos E (2018) Long-term persistent elite HIV-controllers: The right
model of functional cure. EBioMedicine 28: 15-16.
6.
Shasha D, Walker BD (2013) Lessons to be learned
from natural control of HIV - Future directions, therapeutic and preventive
implications. Front Immunol 4: 162.
7.
Kulkarni S, Savan R, Qi Y, Gao X, Yuki Y, et al:
Differential microRNA regulation of HLA-C expression and its association with
HIV control. Nature 472: 495-498.
8.
Miura T, Brumme ZL, Brockman MA, Rosato P, Sela J,
et al. (2010) Impaired replication capacity of acute/early viruses in persons
who become HIV controllers. J Virol 84: 7581-7591.
9.
Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari
G, Giorgi E, et al. (2009) The first T cell response to transmitted/founder
virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med
206: 1253-1272.
10.
Baker BM, Block BL, Rothchild AC, Walker BD (2009)
Elite control of HIV infection: Implications for vaccine design. Expert Opin
Biol Ther 9: 55-69.
11.
Saag M, Deeks SG (2010) How do HIV elite controllers
do what they do? Clin Infect Dis 51: 239-241.
12.
Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K,
et al. (2007) Isolation and characterization of replication-competent human
immunodeficiency virus type 1 from a subset of elite suppressors. J Virol 81: 2508-2518.
13.
Dominguez-Molina B, Leon A, Rodriguez C, Benito JM,
Lopez-Galindez C, et al. (2016) Analysis of non-AIDS-defining events in HIV controllers.
Clin Infect Dis 62: 1304-1309.
14.
Crowell TA, Hatano H (2015) Clinical outcomes and
antiretroviral therapy in 'elite' controllers: A review of the literature. J
Virus Erad 1: 72-77.
15.
Leon A, Perez I, Ruiz-Mateos E, Benito JM, Leal M,
et al. (2016) Rate and predictors of progression in elite and viremic HIV-1
controllers. AIDS 30: 1209-1220.
16.
Reiberger T, Aberle JH, Kundi M, Kohrgruber N,
Rieger A, et al. (2008) IP-10 correlates with hepatitis C viral load, hepatic
inflammation and fibrosis and predicts hepatitis C virus relapse or
non-response in HIV-HCV co-infection. Antiviral Ther 13: 969-976.
17.
Keating SM, Pilcher CD, Jain V, Lebedeva M, Hampton
D, et al. (2017) HIV antibody level as a marker of HIV persistence and
low-level viral replication. J Infect Dis 216: 72-81.
18.
Li JZ, Arnold KB, Lo J, Dugast AS, Plants J, et al.
(2015) Differential levels of soluble inflammatory markers by human
immunodeficiency virus controller status and demographics. Open Forum Infect
Dis 2: ofu117.
19.
Krishnan S, Wilson EM, Sheikh V, Rupert A, Mendoza
D, et al. (2014) Evidence for innate immune system activation in HIV type
1-infected elite controllers. J Infect Dis 209: 931-939.
20.
Gurdasani D, Iles L, Dillon DG, Young EH, Olson AD,
et al. (2014) A systematic review of definitions of extreme phenotypes of HIV
control and progression. AIDS 28: 149-162.
21.
Canoui E, Noel N, Lecuroux C, Boufassa F,
Saez-Cirion A, et al. (2017) Strong ifitm1 expression in CD4 T cells in HIV controllers
is correlated with immune activation. J Acquir Immune Defic Syndr 74: e56-e59.
22.
Dominguez-Molina B, Tarancon-Diez L, Hua S,
Abad-Molina C, Rodriguez-Gallego E, et al. (2017) HLA-B*57 and IFNL4-related
polymorphisms are associated with protection against HIV-1 disease progression
in controllers. J Infect Dis 64: 621-628.
23.
Pernas M, Tarancon-Diez L, Rodriguez-Gallego E,
Gomez J, Prado JG, et al. (2017) Factors leading to the loss of natural elite
control of HIV-1 infection. J Virol.
24.
Sajadi MM, Constantine NT, Mann DL, Charurat M,
Dadzan E, et al. (2009) Epidemiologic characteristics and natural history of
HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr 50: 403-408.
25.
Okulicz JF, Marconi VC, Landrum ML, Wegner S,
Weintrob A, et al. (2009) Clinical outcomes of elite controllers, viremic
controllers and long-term nonprogressors in the US Department of Defense HIV
natural history study. J Infect Dis 200: 1714-1723.
26.
Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland
M, et al. (2008) Relationship between T cell activation and CD4+ T cell count
in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the
absence of therapy. J Infect Dis 197: 126-133.
27.
Chen AK, Sengupta P, Waki K, Van Engelenburg SB,
Ochiya T, et al. (2014) microRNA binding to the HIV-1 Gag protein inhibits Gag
assembly and virus production. Proc Natl Acad Sci U S A 111: E2676-2683.
28.
Crowell TA, Gebo KA, Blankson JN, Korthuis PT, Yehia
BR, et al (2015) Hospitalization rates and reasons among HIV elite controllers
and persons with medically controlled HIV infection. J Infect Dis 211: 1692-1702.
29.
d'Ettorre G, Paiardini M, Zaffiri L, Andreotti M,
Ceccarelli G, et al. (2011) HIV persistence in the gut mucosa of HIV-infected
subjects undergoing antiretroviral therapy correlates with immune activation
and increased levels of LPS. Curr HIV Res 9: 148-153.
30.
Noel N, Boufassa F, Lecuroux C, Saez-Cirion A,
Bourgeois C, et al. (2014) Elevated IP10 levels are associated with immune
activation and low CD4(+) T-cell counts in HIV controller patients. AIDS 28: 467-476.
31.
Platten M, Jung N, Trapp S, Flossdorf P, Meyer-Olson
D, et al. (2016) Cytokine and chemokine signature in elite versus viremic
controllers infected with HIV. AIDS Res Hum Retroviruses 32: 579-587.
32.
Paxton WA, Neumann AU, Kang S, Deutch L, Brown RC,
et al. (2001) RANTES production from CD4+ lymphocytes correlates with host
genotype and rates of human immunodeficiency virus type 1 disease progression. J
Infect Dis 183: 1678-1681.
33.
Kelly MD, Naif HM, Adams SL, Cunningham AL, Lloyd AR
(1998) Dichotomous effects of beta-chemokines on HIV replication in monocytes
and monocyte-derived macrophages. J Immunol 160: 3091-3095.
34.
Liovat AS, Rey-Cuille MA, Lecuroux C, Jacquelin B,
Girault I, et al. (2012) Acute plasma biomarkers of T cell activation set-point
levels and of disease progression in HIV-1 infection. PLoS One 7: e46143.
35.
Njambe Priso GD, Lissom A, Ngu LN, Nji NN, Tchadji
JC, et al. (2018) Filaria specific antibody response profiling in plasma from
anti-retroviral naive Loa loa microfilaremic HIV-1 infected people. BMC Infect Dis
18: 160.
36.
Ambada GN, Ntsama CE, Nji NN, Ngu LN, Sake CN, et al.
(2017) Phenotypic characterization of regulatory T cells from
antiretroviral-naive HIV-1-infected people. Immunology 151: 405-416.
37.
Sake CS, Ngu L, Ambada G, Chedjou JP, Nji N, et al. (2017)
The effect of antiretroviral naïve HIV-1 infection on the ability of natural
killer cells to produce IFN-γ upon exposure to Plasmodium falciparum - Infected erythrocytes. Biomed Hub 2.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Alcoholism Clinical Research
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Spine Diseases
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)