1164
Views & Citations164
Likes & Shares
Transplantation
of donor-derived allogeneic hematopoietic cells causes increased survival in
patients suffering from various blood cancers and other hematologic and
immunologic diseases. However, this health benefit is limited to certain
patients. One major complication is graft-versus-host disease (GVHD) that
occurs when donor-derived immune cells recognize host cells/tissues as foreign
and perpetrate subsequent destruction. Cytokines are a major class of effector
molecules that are involved in GVHD pathogenesis. Proinflammatory cytokines
released by activated immune cells including T cells lead to the onset of GVHD.
T cell depletion (TCD) is an effective approach for GVHD prevention. Several
immune suppressive drugs are also used to treat GVHD. However, these
prophylactic and treatment strategies often lead to an immune compromised state
that increases the risk for infection and cancer relapse. Considering the
adverse effects of TCD and overall immune suppression, more selective
managements such as approaches targeting proinflammatory cytokines have emerged
as a promising strategy to control GVHD. Therefore, this work is dedicated to
review recent development in the studies of cytokines and their future
implication in GVHD therapy.
Keywords: Hematopoietic cell transplantation,
Graft-versus-host disease, T cells, Cytokines
INTRODUCTION
Graft-versus-host
disease (GVHD) is serious complication after allogeneic hematopoietic cell
transplantation (Allo-HCT). During anallo-HCT procedure, hematopoietic cells
harvested from an allogeneic donor are transplanted into a patient who suffers
a certain type of hematologic malignancy or other hematologic or immunologic
disease. After transplantation, however, donor derived immune cells may
recognize normal host cells/tissues as foreign and subsequently cause tissue
damages leading to onset of GVHD. The common symptoms of acute GVHD include
weight loss, alopecia (hair loss on scalp and elsewhere on body), skin lesions,
gastro-intestinal (GI) tract and liver complications that may lead to death
[1]. Among various cellular immune components, T cells are crucially important
in the pathogenesis of GVHD. Donor T cells undergo activation, proliferation
and migration into target organs as a cascade of molecular events involving
interaction between T cell receptors (TCR) and major histocompatibility complex
(MHC) bound allo-antigens, co-stimulatory signaling and cytokine signaling.
Cytokines are immune effectors as well as regulators of various immunologic
conditions including inflammation and hypersensitivity reactions [2,3]. Many
studies have demonstrated that cytokines are involved in various inflammatory
and regulatory activities during GVHD [4,5]. Following encounters between donor
T cells and host antigen presenting cells (APCs) presenting allo-antigens,
allo-reactive donor T cells are activated and undergo vigorous proliferation
and then migrate to the target organs and tissues. These events are accompanied
with enhanced secretion of proinflammatory cytokines that contribute to severe
acute GVHD. That is why a rapid onset of acute GVHD is considered as a result
of “cytokine storm” [6]. The manifestation and severity of GVHD ascribe to naïve
T cells maturing and differentiating into different lineages and phenotypes
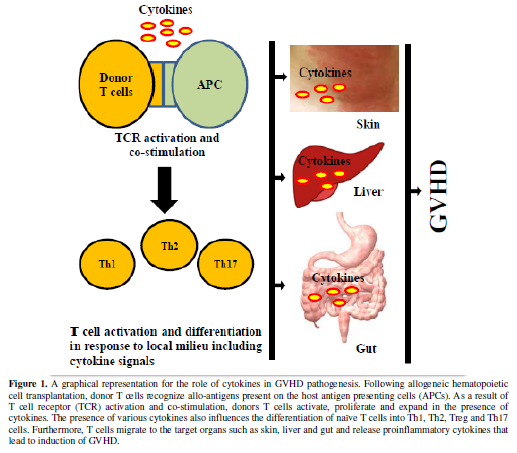
including regulatory T cells (Treg), Th1, Th2, and Th17 cells (Figure 1). This differentiation is
associated with the presence of local cytokines that activate transcription
factors and determine supremacy of certain phenotypes [7]. Th1 cells secret
several cytokines namely interleukin-2 (IL-2), IL-10, interferon-gamma (IFN-γ),
tumor necrosis factor-alpha (TNF-α), and TNF-β. Th2 cells secret cytokines like
IL-3, IL-4, IL-5, IL-10, IL-13, IL-17E and IL-31. Th17 is known to secrets
IL-17 mainly [7]. In GVHD, donor naïve CD4+ T cells recognize allo-antigens
presented on the host’s APCs and differentiated into Th1, Th2, and Th17 cells
depending upon local cytokine milieus. In MHC-mismatched models of GVHD, T
helper (Th) cells differentiated into Th1 subtypes that mediate tissue damage
in GI tract and liver. In the absence of IFN-γ, Th cells differentiated into
Th2 and Th17 subsets and caused damage in lung and skin. If there is neither
IL-4 nor IFN-γ the dominating subset is Th17 cells that lead to tissue damage
in skin. If both the IFN-γ and IL-17 are absent, the dominating subset is Th2
cells that cause idiopathic pneumonia in GVHD patients [8]. Furthermore, in the
presence of IL-12 Th cells differentiate into Th1 phenotype and cause release
of IFN-γ and IL-2 cytokines [9]. In summary, the literature has described
cytokines as effector molecules as well as regulatory factors in GVHD
pathogenesis. This review aims to corroborate the past and current knowledge
regarding the functions of cytokines in GVHD pathogenesis with special emphasis
on potential therapeutic managements.
Th1 Cytokines in GVHD
Among
various Th1 cytokines, IL-2 is one of the most studied cytokines for its role
in activation, proliferation and expansion of T cells in GVHD. Several studies
indicate a potential role of IL-2 in GVHD [10-14]. The role of IL-2 in GVHD is
very diverse that includes amplification of allogeneic immune response,
activation of T cells and NK cells, stimulating secretion of TNF-α by
macrophages and inflammatory damages to the skin and gut [15]. Considering the
importance of IL-2, high dose and low dose of IL-2 therapy are used to diminish
GVHD [11,12]. Administration of high dose IL-2 for several days beginning on
the day of allo-HCT attenuates GVHD mortality in lethally irradiated mice [11].
Low dose therapy with IL-2 has also been reported, that expands Treg population
in vivo and is associated with a lower incidence of GVHD [12]. Low dose of IL-2
restores CD4+Foxp3+Treg homeostasis without causing any
adverse effect on the graft-versus-leukemia/lymphoma (GVL) response [13]. On
the other hand, prevalence of chronic GVHD is characterized by constitutive phosphorylation
of Stat5 in conventional CD4+ T cells (Tcons) associated with elevated amounts
of IL-7 and IL-15 and relative functional deficiency of IL-2. The IL-2 therapy
resulted in the selective increase of Stat5 phosphorylation in Treg and a
decrease of phosphorylated Stat5 in Tcons [14]. However, there are certain
limitations of IL-2 therapy in GVHD. For examples, in experimental GVHD model,
IL-2 administration to donor mice induces a dose-dependent expansion of Treg
cells in the graft but is insufficient to suppress GVHD [16]. In xenogeneic
model of GVHD where human peripheral blood mononuclear cell transplanted into
immunodeficient mice, although, low-dose IL-2 administration caused increase in
Treg cells but was unable to control pro-inflammatory cytokines production by
pathogenic Tcons [16]. Taken together, studies are divided into pro- and
anti-IL2 therapy in GVHD.
Another
member of Th1 secreted cytokines, IL-10 (also known as human cytokine synthesis
inhibitory factor) is a regulatory cytokine that play an important role
in GVHD. IL-10 can modulate CD4+ T cells functions by down-regulation of
another Th1 cytokine IL-2 [17]. IL-10 does not contribute to GVHD mediated by
effector T cells. In contrast, IL-10 creates tolerogenic environment to allo-antigens
independent of IL-2 or CD28 stimulation [18]. A study, using IL-10 deficient
donor or host mice in a MHC-mismatched model of acute GVHD, reported increased
GVHD if either donor or host B cells were unable to produce IL-10 [19]. The induction of IL-10 release in host B
cells attenuates acute GVHD in experimental model [20]. Furthermore, donor bone
marrow graft and Treg-derived IL-10 are important for the donor Treg-mediated
suppression of GVHD [21]. High frequency of donor cells producing IL-10 in response
to host allo-antigen stimulation has been correlated with the absence of acute
GVHD post allo-HCT. Conversely, low frequency of IL-10 response is strongly
associated with more severe GVHD [22].
Another
major Th1 cytokine, IL-12, produced mainly by dendritic cells and macrophages,
is a heterodimeric cytokine that mediated cellular immunity [23]. The dimeric
components of IL-12 are a heavy chain subunit p40 and a light chain subunit p35
that are critical for the functions of active IL-12 [23]. Interestingly, IL-12
shares a common p40 subunit with another cytokine IL-23. This subunit is
important for driving Th1 differentiation and stabilization of Th17 phenotype.
Result showed that both donor- and host-derived p40 is important for the
induction of acute GVHD. The therapeutic efficacy of anti-p40 was evident by
its ability to reduce acute GVHD [24].
Pro-inflammatory
cytokine IFN-γ is mainly produced by activated T-cells, NKT cells and NK
cells. IFN-γ inhibits GVHD in lethally irradiated mice receiving allo-HCT but
promotes lethality in un-irradiated and sub-lethally irradiated recipients
[25]. The extent of conditioning markedly affects the role of IFN-γ in GVHD
lesions mediated by CD4+ T cells. For example, in a GVHD model using sub-lethal
total body irradiation (TBI), the absence of IFN-γ is playing a protective role
in GVHD, while in lethal TBI condition, loss of IFN-γ is associated with
increased pathogenesis [26]. However, there is experimental evidence that IFN-γ
may not be required in GVHD pathogenesis but can facilitate the GVL effects
[27]. Further studies are required to explore IFN-γ involvement in GVHD for
more in depth insight on its use as a therapeutic target.
Another
cytokine from Th1 family that plays important role in the GVHD is TNF-α. It is
directly involved in tissue damages by inducing apoptosis or necrosis of target
cells and synergizes with cytotoxic T lymphocytes and NK cells [28]. Therefore,
use of TNF-α antagonist has shown promising results in GVHD management. For
example, Korngold et al., have investigated that the inhibition of TNF-α during
HCT can diminish inflammatory GVHD reactions without hindering effective GVL
response [28]. However, a recent study reported that TNF-α priming can enhance
CD4+ Foxp3+ regulatory cell suppressive function to
attenuates GVHD [29]. Also, another study indicated that GVHD control depends
to production of TNF-α by T cells and expression of TNFR2 on regulatory T cells
[30].
In
summary, the roles of Th1 cytokines in GVHD pathogenesis are diverse yet not
completely defined. Management of Th1 cytokines for GVHD prophylaxis and
treatment appears promising, but optimization is still an issue that needs
further effort.
Th2 Cytokines in GVHD
Th2
cytokines can attenuate Th1 cytokines such as TNF-α and interrupt Th1 cytokine
cascade post allo-HCT and therefore was once thought to lead to the suppression
of GVHD pathogenesis [31]. Recently, transplantation of myeloid derived
suppressor cells (MDSCs) has been reported to skew allogeneic T cell response
toward Th2 cells and enhanced Th2-specific cytokines that caused suppressed
GVHD [32]. However, other studies have suggested various cytokines of Th2
family are associated with GVHD. For example, IL-3 is a growth promoting
cytokine involved in the differentiation and apoptosis of various hematopoietic
cell types [33]. Up-regulated level of IL-3 is reported in a significant
subgroup of patients suffering from extensive chronic GVHD [34]. Similarly,
high-dosage of IL-3 accelerates GVHD and impairs survival of the host [35].
Another Th2 cytokine, IL-4 is a pleiotropic cytokine produced by activated T
cells [36]. The IL-4 receptor (IL-4R) also binds to IL-13, that contribute to
several overlapping functions of IL-4 and IL-13 [37]. IL-4 plays an important
role in the regulation or pathogenesis of allogeneic responses [38]. Th2 cell
therapy can rapidly ameliorate severe GVHD by creating hindrance in IL-4 and
IL-10 functions, IL-2 consumption and APC modulation [39]. IL-5 is another
member of Th2 cytokine family originally defined as a T-cell-derived cytokine
that triggers activated B cells for terminal differentiation into
antibody-secreting plasma cellsat least in mice [40]. Elevated level of serum
IL-5 is reported in acute GVHD [41]. The cytokines IL-5, IFN-γ, and TNF-α have
been used as biomarkers of acute GVHD [42]. IL-13 is mainly involved in the
allergic inflammation and other ailments including GVHD [3]. Therefore,
association between IL-13 levels and acute GVHD may be exploited as a strong
predictor of this disease. In summary, it is now clear that acute GVHD is not a
purely Th1-type cytokine-driven response, but Th2-type cytokine such as IL-13
has also been involved in the pathogenesis of acute GVHD [42]. Therefore, the
roles of these Th2 cytokines need further mechanistic clarification in GVHD
pathogenesis and management to achieve optimum exploitation.
Th17 Cytokines in GVHD
Th17
cells are known to release cytokines including IL-17and IL-22 [43]. Th17 cells
synergize with naive T cells to induce lethal GVHD. In vitro polarized Th17
cells can induce higher GVHD leading to the severe skin and pulmonary
conditions [44]. Th17 cells have an inverse relationship with Treg cells In
GVHD. The dynamic changes of Th17 and Treg cells along with the level of Th17
cytokines are associated with the onset and resolution of acute GVHD [43].
IL-17 mediates its function via surface receptors on target cells [45].
Participation of IL-17 has been studied in the acute rejection of organ
transplants and GVHD [46]. Both CD4+ IL17-producing T cells and CD8+ IL17-
producing T cells secret the IL-17 cytokine. These subsets of T cells are
suspected to initiate Th1 response at early phase in GVHD [47]. In an allo-HCT
model using pan T cells, IL-17 is dispensable for GVHD and GVL activity.
However, IL-17 contributes to the early development of CD4+ T cell-mediated
GVHD by up-regulating production of proinflammatory cytokines [46]. Concurrent
to this study reports IL‑17‑producing
CD8+ T (Tc17) in the initiation of GVHD [48]. In addition to IL-17, other Th17
cytokines are also targeted by researchers to explore their role in GVHD.
Interleukin-22 protects intestinal stem cells from immune-mediated tissue
damage and regulates sensitivity to GVHD [49]. Treatment
with IL-22 in vivo after allo-HCT enhanced the recovery of intestinal
stem cells, increased epithelial regeneration and reduced intestinal pathology
and mortality from GVHD [50]. In addition, IL-22 producing RORγt+ ILC3 subset
was reported to be involved in the prevention of intestinal GVHD via
strengthening the intestinal mucosal barrier [51]. Furthermore, one of the
other important cytokines that is produced by Th17 and T follicular helper
cells is IL-21 [7]. Also, splenic neutrophils can express IL-21 during acute
GVHD [52]. IL-21 was shown to play a
critical role in GVHD development through increasing B cell activation and
proliferation, alloantibody generation and disrupting Treg homeostasis
[53,54]. It was also shown that direct
or indirect (through Rho associated kinase 2) inhibition of IL-21 ameloraited
GVHD symptoms [55]. Taken together,
these studies show that the diverse roles of Th17 cytokines in GVHD are highly
important, yet more in depth explorations are still required to define the
precise contributions of various Th17 cytokines in GVHD.
Other Cytokines, Chemokines and T Cell
Populations in GVHD
Apart
from Th1, Th2 and Th17 cytokines, various other cytokines also play important
roles in GVHD directly or indirectly. For example, TGF-β-dependent CD103
expression is involved in regulating destruction of gut epithelium by CD8+ T
cells during GVHD pathogenesis [56]. The anti-inflammatory cytokine IL-35 can
suppress acute GVHD in patients post allo-HCT. This cytokine targets
phosphorylation of STAT1 and STAT4 which is generally inhibited in mice in
acute GVHD. Treatment of IL-35 leads to up-regulated phosphorylation of STAT1
and STAT4 and amelioration of acute GVHD. These observations advocate the
potential therapeutic efficacy of IL-35 in GVHD [57]. Homeostatic interleukin
IL-7 regulates T cell survival and proliferation in vivo and is also known as a thymotropic cytokine along with SCF
[58,59]. A recent study suggests that elevated IL-7 but not SCF is associated
with development of GVHD [59]. Another proinflammatory cytokine, IL-15, induces
T cell proliferation and demonstrates IL-2-like properties [60]. Both IL-7 and
IL-15 have been associated with the peripheral T cells regeneration in mice and
humans [61]. Considering the importance of IL-7 and IL-15 in immune functions,
these cytokines have been targeted for the treatment of acute GVHD [61]. In
addition, increased levels of cytokines and chemokines including B cell
activating factor (BAFF), IL-33, CXCL10 and CXCL11 are reported in GVHD
pathogenesis [62]. The role of chemokine CCR7 in GVHD is especially affiliated
with gastrointestinal (GI) tract complications. CCR7 significantly regulates
elevated allo-antigens presentation in mesenteric lymph nodes of GI tract [63].
Furthermore, the binding of IL-33 to receptor “suppression of tumorigenicity 2
(ST2)”presents intriguingly both pro-inflammatory and anti-inflammatory
effects. The increased levels of soluble ST2 are a biomarker for
steroid-refractory GVHD and mortality. Blockade of IL-33 and ST2 interaction
induces marked reduction in GVHD lethality [64]. Another recent study suggests
a role of IL-26 in the pathogenesis of transplant-related obliterative
bronchiolitisas IL-26+CD26+CD4+ T cells in
part induces chronic GVHD of the lungs [1].
Moreover, IL-1β and associated MyD88 signaling in dendritic cells and T
cells are involved in GVHD. After conditioning therapy, the microbial products
and uric acid can activate NLRP-3 in donor T cells to increase IL-1β expression
that subsequently enhances GVHD severity [65]. On the other hand, it has been
demonstrated that MyD88 deficiency in T-cell depleted donor BM leads to
attenuated GVHD symptoms [66].
In
addition to Th1, Th2 and Th17 cells that produce associated cytokines and
affect GVHD, there are several important T cell populations that also impact
GVHD via cytokines and other mechanisms.
Many studies have documented the roles of Treg cells in GVHD. Both natural and induced Treg cells are able
to restrain conventional T cell proliferation and attenuate GVHD with multiple
mechanisms including IL-2, IL-10 and TGF-β [67]. CD4+CD103+Fop3+natural
Treg can directly migrate to GVHD target organs decreasing disease severity
[68]. Notably, the Th17/Treg ratio is correlated with clinical and pathological
GVHD and therefore could be used as a biomarker of GVHD [69]. IL-2 treatment in combination with rapamycin
has been shown to mitigate acute GVHD lethality, which is associated with
increased expansion of donor-type CD4⁺Foxp3⁺
Treg cells and reduction of CD4⁺CD25⁻
conventional T cells [67]. In addition,
T follicular helper (Tfh) cells are responsible for naïve B cells differentiation
to memory B cells and immunoglobulin class switching. Tfh cells express BCL-6
as transcription factor and CXCR5 and PD-1 surface markers with high secretion
of IL-21 cytokine. Tfh cells mostly locate in germinal center but a subset of
Tfh cells have been detected in peripheral blood. Tfh cells are required for
generation and maintenance of germinal center and B cells function in chronic
GVHD. These circulating Tfh cells appear to cause aggravation of chronic GVHD
[70,71]. Furthermore, a more recent
study has described a new CD4+ memory T population with high
expression of CD11c and α4β7 in gut that plays a pivotal role in initiating
gastrointestinal GVHD via promoting Th1 response and cytokine production [72].
Taken
together, these studies indicate that a wide range of cytokines and T cell
populations are involved in the pathogenesis of GVHD. A thorough understanding
of the complex molecular and cellular networks will provide better insights for
more effective GVHD management.
Updates on Cytokine-Related GVHD Management
Strategies
Recent
years witnessed a significant growth in findings related to the role of various
cytokines in the pathogenesis and management of GVHD. Several new drugs have
been used to treat GVHD based on cytokine regulation. For example, zinc
supplementation was reported to be beneficial in the induction of tolerance
that amelioratesTh1-dominated allogeneic immune response [73]. A recent study
also advocates granzyme B (GzmB) based therapeutic approach because GzmB knockout
T cells are associated with the production of prominent quantity of
proinflammatory cytokines that exacerbated GVHD [74]. An in vitro study using dendritic cell (DC) culture indicated that
bortezomib can inhibit the proliferation of DCs and also blocked expression of
costimulatory molecules CD80 and CD86. This drug was also found to diminish
IL-12 and TNF-α release in DCs following treatment of LPS [75]. Another study
was carried out on valproic acid, a histone deacetylase inhibitor that also
possesses anti-inflammatory effects. In
MHC-mismatched transplantation mouse model, valproic acid down regulated
Th1 and Th17 cell responses and cytokine production in vitro and in vivo
[76]. The use cyclopentylamino carboxymethylthiazolylindole-7 (NecroX-7) is
found useful in controlling GVHD in preclinical models. NecroX-7 is an
inhibitor of chromatin protein high mobility group box 1 (HMGB1). NecroX-7
protects mice against lethal GVHD by reciprocal regulation of Treg/Th1 cells
[77]. Erlotinib, an EGFR tyrosine kinase inhibitor ameliorates sclerodermatous
GVHD. These beneficial effects were mediated by decrease in IFN-γ and IL-13
production and autoimmune B-cell activation [78]. Moreover, BET bromodomain
inhibition can suppress GVHD through NF-κB regulation and decrease production
of inflammatory cytokines such as IL-6, IL-12, TNF-α in DCs and IFN-γ, IL-2,
IL-4 and IL-17 in activated T cells [79]. Although initial outcomes of these
cytokine-related therapeutic regimens raise hopes for better treatment, further
study is warranted to achieve optimum benefits.
Micro
RNAs (miRs) play important roles in regulation of various immune responses such
as infection, tumor, and autoimmunity [80]. Recently, the function of miR-17-92
cluster in allogeneic T cell response has been studied. Results suggest an
important role for miR-17-92 in donor T cells for GVHD induction. The miR-17-92
promotes CD4+ T cell functions, Th1 differentiation, but down-regulates Th2 and
inducible Treg functions [80]. In addition, miR-142 is implicated in hematopoietic
functions including T cell response. In
vivo deletion of miR-142 does not affect T cell development. But in vitro
and multiple models of GVHD targeting miR-142 leads to attenuated T cell
proliferation [81]. Recently, miR-155, known to regulate the innate immune
system, is studied for its role in DC functions during GVHD. Study suggests
that miR-155 deficiency in host system is associated with decreased
pro-inflammatory cytokines and attenuated GVHD pathogenesis [82]. Moreover, an
earlier study showed that expression of
miR146a increased in donor T cells after transplantation and deficiency
in Mir146a caused enhanced GVHD through TRAF6/TNF signaling pathway [83]. These
studies suggest that genetic manipulation by targeting miRs in GVHD therapy may
have beneficial impacts.
Uses
agonistic antibodies have shown proved efficacy in GVHD management. Recently,
αDR3, an antibody to death receptor 3 (DR3), has been investigated for its role
in the management of GVHD. DR3 is mainly present on Treg cells, lymphoid tissue
inducer cells and NKT cells [84]. Studies showed that agonistic antibody αDR3
expanded CD4+FoxP3+ Treg cells population in
vivo. Apart from the expansion of Treg cells, αDR3 also down-regulated
proinflammatory cytokines such as IFN-γ, IL-1β, and TNF-α. Notably this GVHD
alleviating effect was achieved by a single dose of αDR3 [84]. Another target
aimed to alleviate GVHD is the TNF-like weak inducer of apoptosis
(TWEAK)/fibroblast growth factor-inducible 14 (Fn14) axis. It was observed that
attenuation of TWEAK/Fn14 system alleviated disease development in several
models of colitis [85]. In GVHD, proinflammatory cytokine TNF play very
important role in intestinal cell death. TWEAK up-regulates TNF-induced cell
death. Therefore, proper understanding of Fn14 in TNF associated GVDH
pathogenesis will be useful. As such, an antibody-dependent cell-mediated
cytotoxicity (ADCC)-defective Fn14-blocking antibody has been utilized by
researchers to attenuate GVHD successfully [85]. These studies advocate
promising roles of monoclonal antibodies in GVHD management.
Cytokines
also find uses as biomarkers for GVHD. For example, tear cytokine profile was
found associated with systemic chronic GVHD. This finding is evident by the
presence of enhanced levels of IL-2, IL-10, IL-17α, IFN-γ, IL-6, and TNF-α tear
cytokines in chronic GVHD patients [86]. Recently, IL-6 and IL-9 have been
studied for their potential as biomarkers in GVHD [87]. In addition to
modulating T cells, recent study has also shown a potential role of cytokines
in modulation of NK cells. Adoptive transfer of IL-12, IL-15 and IL-18
pre-activated NK cells showed suppression of GVHD in a mouse model of
MHC-mismatched HCT [88].Together, adoptive transfer of cytokines pretreated immune
cells will pose a potential impact on the GVHD management.
CONCLUDING REMARK
In
summary, the role of cytokines in GVHD is indispensable. Several therapeutic
interventions based on targeting cytokines have already shown promising results
in GVHD amelioration. As Th1, Th2 and Th17 cytokines are associated with the
pathogenesis and management, exploitation of these immune mediators will
produce practical approaches to combat GVHD. Further studies are required to
understand the underlying mechanisms associated with the roles of cytokines in
GVHD in order to achieve optimum diagnostic and therapeutic benefits.
CONFLICT OF INTEREST
Authors
have declared that there is no conflict of interest.
ACKNOWLEDGEMENT
This work
was supported by NIH research grant # R01CA184728
(X.C.). We apologize to the authors whose studies are relevant to this subject
but are not cited due to space limitation.
- Ohnuma K, Hatano R, Aune TM,
Otsuka H, Iwata S, et al. (2015) Regulation of pulmonary graft-versus-host
disease by IL-26+CD26+CD4 T lymphocytes. J Immunol 194: 3697-3712.
- Kelso A (1998) Cytokines:
principles and prospects. Immunol Cell Biol 76: 300-317.
- Kumar S, Verma AK, Das M,
Dwivedi PD (2012) Molecular mechanisms of IgE mediated food allergy. Int
Immunopharmacol 13: 432-439.
- Ferrara JL (1993) Cytokine
dysregulation as a mechanism of graft versus host disease. Curr Opin
Immunol 5: 794-799.
- Fujii N, Hiraki A, Aoe K,
Murakami T, Ikeda K, et al. (2006) Serum cytokine concentrations and acute
graft-versus-host disease after allogeneic peripheral blood stem cell
transplantation: concurrent measurement of ten cytokines and their
respective ratios using cytometric bead array. Int J Mol Med 17: 881-885.
- Mohty M, Blaise D, Faucher C,
Vey N, Bouabdallah R, et al. (2005) Inflammatory cytokines and acute
graft-versus-host disease after reduced-intensity conditioning allogeneic
stem cell transplantation. Blood 106: 4407-4411.
- Henden AS, Hill GR (2015)
Cytokines in Graft-versus-Host Disease. J Immunol 194: 4604-4612.
- Yi T, Chen Y, Wang L, Du G,
Huang D, et al. (2009) Reciprocal differentiation and tissue-specific
pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease.
Blood 114: 3101-3112.
- Kichian K, Nestel FP, Kim D,
Ponka P, Lapp WS (1996) IL-12 p40 messenger RNA expression in target
organs during acute graft-versus-host disease. Possible involvement of
IFN-gamma. J Immunol 157: 2851-2856.
- Shindo T, Ishikawa T,
Fukunaga A, Hori T, Uchiyama T (2008) Growth and differentiation
advantages of CD4+ OX40+ T cells from allogeneic hematopoietic stem cell
transplantation recipients. Biol Blood Marrow Transplant 14: 268-281.
- Sykes M, Romick ML, Sachs DH
(1990) Interleukin 2 prevents graft-versus-host disease while preserving
the graft-versus-leukemia effect of allogeneic T cells. Proc Natl Acad Sci
U S A 87: 5633-5637.
- Kennedy-Nasser AA, Ku S,
Castillo-Caro P, Hazrat Y, Wu MF, et al. (2014) Ultra low-dose IL-2 for
GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation
mediates expansion of regulatory T cells without diminishing antiviral and
antileukemic activity. Clin Cancer Res 20: 2215-2225.
- Sykes M, Harty MW, Szot GL,
Pearson DA (1994) Interleukin-2 inhibits graft-versus-host
disease-promoting activity of CD4+ cells while preserving CD4- and
CD8-mediated graft-versus-leukemia effects. Blood 83: 2560-2569.
- Matsuoka K, Koreth J, Kim HT,
Bascug G, McDonough S, et al. (2013) Low-dose interleukin-2 therapy
restores regulatory T cell homeostasis in patients with chronic
graft-versus-host disease. Sci Transl Med 5: 179ra143.
- Ball LM, Egeler RM; EBMT
Paediatric Working Party (2008) Acute GvHD: pathogenesis and
classification. Bone Marrow Transplant 41 Suppl 2: S58-64.
- Pérol L, Martin GH, Maury S,
Cohen JL, Piaggio E (2014) Potential limitations of IL-2 administration
for the treatment of experimental acute graft-versus-host disease. Immunol
Lett 162: 173-184.
- Taga K, Mostowski H, Tosato G
(1993) Human interleukin-10 can directly inhibit T-cell growth. Blood 81:
2964-2971.
- Groux H, Bigler M, de Vries
JE, Roncarolo MG (1996) Interleukin-10 induces a long-term
antigen-specific anergic state in human CD4+ T cells. J Exp Med 184:
19-29.
- Weber M, Stein P, Prüfer S,
Rudolph B, Kreft A, et al. (2014) Donor and host B cell-derived IL-10
contributes to suppression of graft-versus-host disease. Eur J Immunol 44:
1857-1865.
- Rowe V, Banovic T, MacDonald
KP, Kuns R, Don AL et al. (2006) Host B cells produce IL-10 following TBI
and attenuate acute GVHD after allogeneic bone marrow transplantation.
Blood 108: 2485-2492.
- Tawara I, Sun Y, Liu C,
Toubai T, Nieves E, et al. (2012) Donor- but not host-derived
interleukin-10 contributes to the regulation of experimental
graft-versus-host disease. J Leukoc Biol 91: 667-675.
- Weston LE, Geczy AF, Briscoe
H (2006) Production of IL-10 by alloreactive sibling donor cells and its
influence on the development of acute GVHD. Bone Marrow Transplant 37:
207-212.
- Gately MK, Renzetti LM,
Magram J, Stern AS, Adorini L, et al. (1998) The
interleukin-12/interleukin-12-receptor system: role in normal and
pathologic immune responses. Annu Rev Immunol 16: 495-521.
- Wu Y, Bastian D, Schutt S,
Nguyen H, Fu J, et al. (2015) Essential Role of Interleukin-12/23p40 in
the Development of Graft-versus-Host Disease in Mice. Biol Blood Marrow
Transplant 21: 1195-1204.
- Wang H, Asavaroengchai W,
Yeap BY, Wang MG, Wang S, et al. (2009) Paradoxical effects of IFN-gamma
in graft-versus-host disease reflect promotion of lymphohematopoietic graft-versus-host
reactions and inhibition of epithelial tissue injury. Blood 113:
3612-3619.
- Welniak LA, Blazar BR, Anver
MR, Wiltrout RH, Murphy WJ (2000) Opposing roles of interferon-gamma on
CD4+ T cell-mediated graft-versus-host disease: effects of conditioning.
Biol Blood Marrow Transplant 6: 604-612.
- Yang YG, Wang H,
Asavaroengchai W, Dey BR (2005) Role of Interferon-gamma in GVHD and GVL.
Cell Mol Immunol 2: 323-329.
- Korngold R, Marini JC, de
Baca ME, Murphy GF, Giles-Komar J (2003) Role of tumor necrosis
factor-alpha in graft-versus-host disease and graft-versus-leukemia
responses. Biol Blood Marrow Transplant 9: 292-303.
- Pierini A, Strober W, Moffett
C, Baker J, Nishikii H, et al. (2016) TNF-alpha priming enhances
CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD
prevention and treatment. Blood 128: 866-871.
- Leclerc M, Naserian S, Pilon
C, Thiolat A, Martin GH, et al. (2016) Control of GVHD by regulatory T
cells depends on TNF produced by T cells and TNFR2 expressed by regulatory
T cells. Blood 128: 1651-1659.
- Krenger W, Ferrara JL (1996)
Graft-versus-host disease and the Th1/Th2 paradigm. Immunol Res 15: 50-73.
- Messmann JJ, Reisser T,
Leithauser F, Lutz MB, Debatin KM (2015) In vitro-generated MDSCs prevent
murine GVHD by inducing type 2 T cells without disabling antitumor
cytotoxicity. Blood 126: 1138-1148.
- Korpelainen EI, Gamble JR,
Vadas MA, Lopez AF (1996) IL-3 receptor expression, regulation and function
in cells of the vasculature. Immunol Cell Biol 74: 1-7.
- Valent P, Sillaber KC,
Scherrer R, Geissler K, Kier P, et al. (1992) Detection of circulating
endogenous interleukin-3 in extensive chronic graft-versus-host disease.
Bone Marrow Transplant 9: 331-336.
- Atkinson K, Vos B, Kang-Er Z,
Guiffre A, Seymour R, et al. (1995) Effect of in vivo administration of
IL-3 and IL-6, alone and in combination with G-CSF, GM-CSF or IL-1, on
haematopoiesis, graft-versus-host disease and survival after murine
haematopoietic stem cell transplantation. Cytokines Mol Ther 1: 47-55.
- Paul WE (1991) Interleukin-4:
a prototypic immunoregulatory lymphokine. Blood 77: 1859-1870.
- Andrews AL, Holloway JW,
Holgate ST, Davies DT (2006) IL-4 receptor alpha is an important modulator
of IL-4 and IL-13 receptor binding: implications for the development of
therapeutic targets. J Immunol 176: 7456-7461.
- Ushiyama C, Hirano T,
Miyajima H, Okumura K, Ovary Z, et al. (1995) Anti-IL-4 antibody prevents
graft-versus-host disease in mice after bone marrow transplantation. The
IgE allotype is an important marker of graft-versus-host disease. J
Immunol 154: 2687-2696.
- Foley JE, Mariotti J, Ryan K,
Eckhaus M, Fowler DH (2008) Th2 cell therapy of established acute
graft-versus-host disease requires IL-4 and IL-10 and is abrogated by IL-2
or host-type antigen-presenting cells. Biol Blood Marrow Transplant 14:
959-972.
- Kouro T, Takatsu K (2009)
IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int
Immunol 21: 1303-1309.
- Imoto S, Oomoto Y, Murata K,
Das H, Murayama T, et al. (2000) Kinetics of serum cytokines after
allogeneic bone marrow transplantation: interleukin-5 as a potential
marker of acute graft-versus-host disease. Int J Hematol 72: 92-97.
- Jordan WJ, Brookes PA, Szydlo
RM, Goldman JM, Lechler RI, et al. (2004) IL-13 production by donor T
cells is prognostic of acute graft-versus-host disease following unrelated
donor stem cell transplantation. Blood 103: 717-724.
- Liu Y, Cai Y, Dai L, Chen G,
Ma X, et al. (2013) The expression of Th17-associated cytokines in human
acute graft-versus-host disease. Biol Blood Marrow Transplant 19:
1421-1429.
- Carlson MJ, West ML, Coghill
JM, Panoskaltsis-Mortari A, Blazar BR, et al. (2009) In
vitro-differentiated TH17 cells mediate lethal acute graft-versus-host
disease with severe cutaneous and pulmonary pathologic manifestations.
Blood 113: 1365-1374.
- Korn T, Bettelli E, Oukka M,
Kuchroo VK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27: 485-517.
- Kappel LW, Goldberg GL, King
CG, Suh DY, Smith OM, et al. (2009) IL-17 contributes to CD4-mediated
graft-versus-host disease. Blood 113: 945-952.
- van der Waart AB, van der
Velden WJ, Blijlevens NM, Dolstra H (2014) Targeting the IL17 pathway for
the prevention of graft-versus-host disease. Biol Blood Marrow Transplant
20: 752-759.
- Zhao K, Ruan S, Yin L, Zhao
D, Chen C, et al. (2016) Dynamic regulation of effector IFN-γ-producing
and IL-17-producing T cell subsets in the development of acute
graft-versus-host disease. Mol Med Rep 13: 1395-1403.
- Hanash AM, Dudakov JA, Hua G,
O'Connor MH, Young LF, et al. (2012) Interleukin-22 protects intestinal
stem cells from immune-mediated tissue damage and regulates sensitivity to
graft versus host disease. Immunity 37: 339-350.
- Lindemans CA, Calafiore M,
Mertelsmann AM, O'Connor MH, Dudakov JA, et al. (2015) Interleukin-22
promotes intestinal-stem-cell-mediated epithelial regeneration. Nature
528: 560-564.
- Konya V, Mjösberg J (2015)
Innate lymphoid cells in graft-versus-host disease. Am J Transplant 15:
2795-2801.
- Thompson JS, Hardin DL, Glass
JF3, Dziba J3, Campion J4, et al. (2016) The Inflammatory Cytokine IL-21
is Expressed by Splenic Neutrophils in Response to Transplantation of
Allogeneic Cells. SOJ Immunol 4: 1-9.
- Wu X, Tan Y, Xing Q, Wang S
(2013) IL-21 accelerates xenogeneic graft-versus-host disease correlated
with increased B-cell proliferation. Protein Cell 4: 863-871.
- Lim JY, Park MJ, Im KI, Kim
N, Park HS, et al. (2015) Interleukin 21 blockade modulates activated T-
and B-cell homeostasis via B-cell activating factor pathway-mediated
inhibition in a murine model of acute graft-versus-host disease. Exp
Hematol 43: 23-31 e21-22.
- Flynn R, Paz K, Du J,
Reichenbach DK, Taylor PA, et al. (2016) Targeted Rho-associated kinase 2
inhibition suppresses murine and human chronic GVHD through a
Stat3-dependent mechanism. Blood 127: 2144-2154.
- El-Asady R., Yuan R, Liu K,
Wang D, Gress RE, et al. (2005) TGF-{beta}-dependent CD103 expression by
CD8(+) T cells promotes selective destruction of the host intestinal epithelium
during graft-versus-host disease. J Exp Med 201: 1647-1657.
- Zhang XH, Zhou Y, Zhang JM,
Zhou SY, Wang M, et al. (2015) IL-35 inhibits acute graft-versus-host
disease in a mouse model. Int Immunopharmacol 29: 383-392.
- Bazdar DA, Kalinowska M, Panigrahi
S, Sieg SF (2015) Recycled IL-7 Can Be Delivered to Neighboring T Cells. J
Immunol 194: 4698-4704.
- Politikos I, Kim HT,
Nikiforow S, Li L, Brown J, et al. (2015) IL-7 and SCF Levels Inversely
Correlate with T Cell Reconstitution and Clinical Outcomes after Cord
Blood Transplantation in Adults. PLoS One 10: e0132564.
- Fehniger TA, Caligiuri MA
(2001) Interleukin 15: biology and relevance to human disease. Blood 97:
14-32.
- Thiant S, Moutuou MM, Leboeuf
D, Guimond M (2016) Homeostatic cytokines in immune reconstitution and
graft-versus-host disease. Cytokine 82: 24-32.
- Ahmed SS, Wang XN, Norden J,
Pearce K, El-Gezawy E, et al. (2015) Identification and validation of
biomarkers associated with acute and chronic graft versus host disease.
Bone Marrow Transplant 50: 1563-1571.
- Koyama M, Cheong M, Markey
KA, Gartlan KH, Kuns RD, et al. (2015) Donor colonic CD103+ dendritic
cells determine the severity of acute graft-versus-host disease. The
Journal of experimental medicine 212: 1303-1321.
- Reichenbach DK, Schwarze V,
Matta BM, Tkachev V, Lieberknecht E, et al. (2015) The IL-33/ST2 axis
augments effector T-cell responses during acute GVHD. Blood 125:
3183-3192.
- Jankovic D, Ganesan J,
Bscheider M, Stickel N, Weber FC, et al. (2013) The Nlrp3 inflammasome regulates
acute graft-versus-host disease. J Exp Med 210: 1899-1910.
- Lim JY, Lee YK, Lee SE, Ju
JM, Park G, et al. (2015) Attenuation of Hepatic Graft-versus-host Disease
in Allogeneic Recipients of MyD88-deficient Donor Bone Marrow. Immune Netw
15: 125-134.
- Shin HJ, Baker J,
Leveson-Gower DB, Smith AT, Sega EI, et al. (2011) Rapamycin and IL-2
reduce lethal acute graft-versus-host disease associated with increased
expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood 118:
2342-2350.
- Zhao D, Zhang C, Yi T, Lin
CL, Todorov I, et al. (2008) In vivo-activated CD103+CD4+ regulatory T
cells ameliorate ongoing chronic graft-versus-host disease. Blood 112:
2129-2138.
- Ratajczak P, Janin A,
Peffault de Latour R, Leboeuf C, Desveaux A, et al. (2010) Th17/Treg ratio
in human graft-versus-host disease. Blood 116: 1165-1171.
- Forcade E, Kim HT, Cutler C,
Wang K, Alho AC, et al. (2016) Circulating T follicular helper cells with
increased function during chronic graft-versus-host disease. Blood 127:
2489-2497.
- Flynn R, Du J, Veenstra RG,
Reichenbach DK, Panoskaltsis-Mortari A, et al. (2014) Increased T
follicular helper cells and germinal center B cells are required for cGVHD
and bronchiolitis obliterans. Blood 123: 3988-3998.
- Zhou V, Agle K, Chen X, Beres
A, Komorowski R, et al. (2016) A colitogenic memory CD4+ T cell population
mediates gastrointestinal graft-versus-host disease. J Clin Inv 126:
3541-3555.
- Rosenkranz E, Metz CH,
Maywald M, Hilgers RD, Weßels I, et al. (2016) Zinc supplementation
induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed
lymphocyte cultures. Mol Nutr Food Res 60: 661-671.
- Du W, Leigh ND, Bian G,
O'Neill RE, Mei L, et al. (2015) Granzyme B-Mediated Activation-Induced
Death of CD4+ T Cells Inhibits Murine Acute Graft-versus-Host Disease. J
Immunol 195: 4514-4523.
- Wang Y, Liang Y, Zhang Y, Wu
D, Liu H (2015) Bortezomib inhibits bone marrow-derived dendritic cells.
Int J Clin Exp Pathol 8: 4857-4862.
- Long J, Chang L, Shen Y, Gao
WH, Wu YN, et al. (2015) Valproic Acid Ameliorates Graft-versus-Host
Disease by Downregulating Th1 and Th17 Cells. J Immunol 195: 1849-1857.
- Im KI, Kim N, Lim JY, Nam YS,
Lee ES, et al. (2015) The Free Radical Scavenger NecroX-7 Attenuates Acute
Graft-versus-Host Disease via Reciprocal Regulation of Th1/Regulatory T
Cells and Inhibition of HMGB1 Release. J Immunol 194: 5223-5232.
- Morin F, Kavian N, Marut W,
Chereau C, Cerles O, et al. (2015) Inhibition of EGFR Tyrosine Kinase by
Erlotinib Prevents Sclerodermatous Graft-Versus-Host Disease in a Mouse
Model. J Invest Dermatol 135: 2385-2393.
- Sun Y, Wang Y, Toubai T,
Oravecz-Wilson K, Liu C, et al. (2015) BET bromodomain inhibition
suppresses graft-versus-host disease after allogeneic bone marrow
transplantation in mice. Blood 125: 2724-2728.
- Wu Y, Heinrichs J, Bastian D,
Fu J, Nguyen H, et al. (2015) MicroRNA-17-92 controls T-cell responses in
graft-versus-host disease and leukemia relapse in mice. Blood 126:
1314-1323.
- Sun Y, Oravecz-Wilson K,
Mathewson N, Wang Y, McEachin R, et al. (2015) Mature T cell responses are
controlled by microRNA-142. J Clin Invest 125: 2825-2840.
- Chen S, Smith BA, Iype J,
Prestipino A, Pfeifer D, et al. (2015) MicroRNA-155-deficient dendritic
cells cause less severe GVHD through reduced migration and defective
inflammasome activation. Blood 126: 103-112.
- Stickel N, Prinz G, Pfeifer
D, Hasselblatt P, Schmitt-Graeff A, et al. (2014) MiR-146a regulates the
TRAF6/TNF-axis in donor T cells during GVHD. Blood 124: 2586-2595.
- Kim BS, Nishikii H, Baker J,
Pierini A, Schneidawind D, et al. (2015) Treatment with agonistic DR3
antibody results in expansion of donor Tregs and reduced graft-versus-host
disease. Blood 126: 546-557.
- Chopra M, Brandl A, Siegmund
D, Mottok A, Schafer V, et al. (2015) Blocking TWEAK-Fn14 interaction
inhibits hematopoietic stem cell transplantation-induced intestinal cell
death and reduces GVHD. Blood 126: 437-444.
- Jung JW, Han SJ, Song MK, Kim
TI, Kim EK, et al. (2015) Tear Cytokines as Biomarkers for Chronic
Graft-versus-Host Disease. Biol Blood Marrow Transplant 21: 2079-2085.
- Cocho L, Fernandez I, Calonge
M, Martinez V, Gonzalez-Garcia MJ, et al. (2015) Gene Expression-Based
Predictive Models of Graft Versus Host Disease-Associated Dry Eye. Invest
Ophthalmol Vis Sci 56: 4570-4581.
- Hüber CM, Doisne JM, Colucci
F (2015) IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model
of mismatched hematopoietic cell transplantation. Eur J Immunol 45:
1727-1735.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Spine Diseases
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Dermatology Clinics and Research (ISSN:2380-5609)