1030
Views & Citations30
Likes & Shares
Cutaneous leishmaniasis (CL) a public health issue which is associated
with considerable morbidity is still a major health problem in the world
especially in developing countries Nigeria inclusive. The study is aimed at
determine the prevalence of Cutaneous leishmaniasis. Data was collected from
the health facility client’s sixty three (63) (leishmania suspect) at the
outpatient department within the period of June to November 2018. leishmaniasis
was diagnosed microscopically by staining thick and thin blood films on a glass
slide, to visualize amastigote and promastigote of parasites intracellularly or
extracellularly. Result showed that Among the 63 leishmaniasis suspect from the
out-patient department for screening test, 46 (73%) tested positive to
leishmaniasis (amastigote) microscopically. Among 46 leishmaniasis positive
clients 29 (63%) were male and 17 (37%) were female. The age with higher
prevalence was ≥ 15 years with 39 (85%) client while age ≤ 15 years were 7
(15%). Cutaneous leishmaniasis exists in Sokoto State in high proportion.
Keywords: Cutaneous
leishmaniasis, Leison, Amastigote, Prevalence, Papule
INTRODUCTION
Cutaneous leishmaniasis (also known as
oriental sore, tropical sore, chiclero ulcer, chiclero's ulcer or Aleppo boil,
“Delhi Boil”) [1,2]. Institute for International Cooperation in Animal
Biologics and the Center for Food Security and Public Health [2], is the most
common form of leishmaniasis affecting humans [3]. It is a skin infection
caused by a single-celled parasite that is transmitted by the bite of a
phlebotomine sandfly. There are about twenty species of Leishmania that may
cause cutaneous leishmaniasis. This disease is considered to be a zoonosis (an
infectious disease that is naturally transmissible from vertebrate animals to
humans), with the exception of Leishmania
tropica — which is often an anthroponotic disease (an infectious disease
that is naturally transmissible from humans to vertebrate animals) [2].
Cutaneous leishmaniasis is endemic in all tropical and subtropical areas of the
world [4]. The distribution of this disease is very tightly
linked to
JUSTIFICATION OF THE STUDY
Cutaneous
leishmaniasis is endemic in all tropical and subtropical areas of the world
[4]. The huge increase in the spread of the disease is attributed to the
refugee crises in the Middle East and West and North Africa over the past five
years, particularly due to the displacement of millions of Syrian refugees and
in Africa [13]. The outbreak among Syrian refugees and Sudan was documented by
the World Health Organization (WHO) in 2012 and recognized as ongoing [14]. The
Middle East, in 2016, seems to be experiencing an increase in the cutaneous
leishmaniasis disease due to migrants fleeing the Islamic State of Iraq and the
Levant. Reports of the increase in the disease have surfaced in Turkey, Lebanon
and elsewhere [15,16].
OBJECTIVE OF THE STUDY
The aim and objectives
of this study were therefore to determine the prevalence of leishmaniasis in
Sokoto, North Western Nigeria.
SIGNIFICANCE OF THE STUDY
Results of the study
would reveal the prevalence of leishmaniasis in Sokoto metropolis.
Specifically, result of the study would be significant to adults (male/female),
Public health officers, health counselors, health educators, curriculum
planners, medical allied personnel and researchers in assessing the prevalence
malaria disease and initiating preventive measures in among inhabitants would
help prevention programs succeed in the populace in Sokoto metropolis.
RESEARCH QUESTIONS
·
What is prevalence of leishmaniasis case
in the facility?
·
What is prevalence of leishmaniasis
cases among children <15 years?
·
What is the number of leishmaniasis case
among female and male in general patients and children less than fifteen years?
HYPOTHESES
·
There is no prevalence of leishmaniasis
case in the facility.
·
There is no leishmaniasis case among
children <15 years.
·
There is no leishmaniasis case among
female and male in general patients and children less than fifteen years.
MATERIALS AND METHODS
Study area and the population
1Brigade Medical
Centre, Gingiya barracks, Dange Shuni LGA in Sokoto South senatorial zone was
be taken as study areas. By the virtue of its origin, the state comprises
mostly Hausa/Fulani and other groups such as Gobirawa, Zabarmawa, Kabawa,
Adarawa, Arawa, Nupes, Yorubas, Igbos and others. The Sokoto township is in dry
Sahel surrounded by sandy terrain and isolated hills. Rainfall starts late that
is in June and ends in September but may sometimes extend into October. The
average annual rainfall is 550 mm with peak in the month August. The highest
temperatures of 45°C during the hot season are experienced in the months of
March and April. Harmattan a dry, cold and dusty condition is experienced
between the months of November and February.
Ethical approval
This research will get
ethical clearance from the Ethical Committee of the 1Brigade Medical Centre,
Ginginya barrack, Sokoto and seek permission for collection data. This study
was conducted in accordance with the Declaration of Helsinki and the protocol
was approved by the ethical research committee of the 1Brigade medical centre,
Ginginya barrack, Sokoto.
Sample size
The sample size was
sixty three (63) leishmaniasis suspects at out- patients department that
attended the facility between the months of June to November, 2018.
Method of data collection
The data was collected
from the health facility client’s (leishmania suspect) at the outpatient
department within the period of June to November 2018. leishmaniasis was
diagnosed microscopically by staining thick and thin blood films on a glass
slide, to visualize amastigote and promastigote of parasites intracellularly or
extracellularly. Briefly, the client’s lesion/sore was cleaned with 70% ethyl
alcohol, allowed to dry and then the lesion smears of each active lesion
observed was prepared for microscopy on a glass slide. To prepare a thick blood
film, a blood spot was stirred in a circular motion with the corner of the
slide, taking care not make the preparation too thick and allowed to dry
without fixative. After drying, the spot was stained with diluted Giemsa (1:20,
v/v) for 20 min and washed by placing the film in buffered water for 3 min. The
slide is allowed to air-dry in a vertical position and examination using a
light microscope. As they are unfixed, the red cells lyse when a water-based
stain is applied. A thin blood film was prepared by immediately placing the smooth
edge of a spreader slide in a drop of blood, adjusting the angle between slide
and spreader to 45° and then smearing the blood with a swift and steady sweep
along the surface. The film was then allowed to air-dry and is fixed with
absolute methanol. After drying, the sample is stained with diluted Giemsa
(1:20, v/v) for 20 min and washed by briefly dipping the slide in and out of a
jar of buffered water (excessive washing will decolorize the film). The slide
was then allowed to air-dry in a vertical position and examined under a light
microscope (100x objectives) [17].
Method of data analysis
Data collected were
analyzed using descriptive statistic of frequency count, normative percentage.
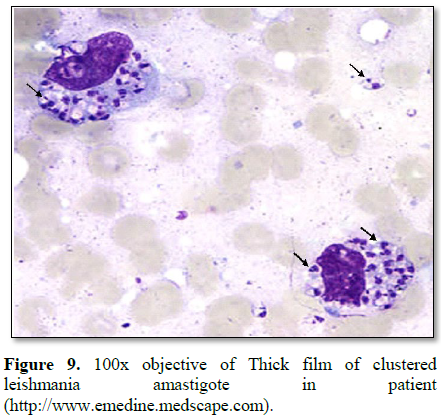
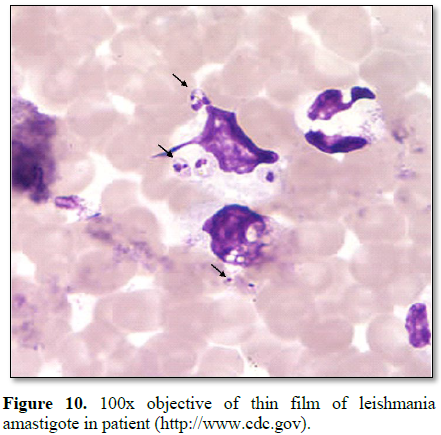
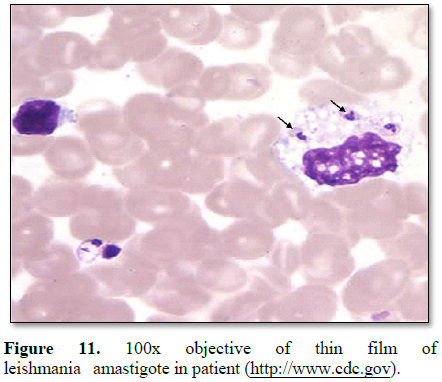
RESULTS
Among the 63
leishmaniasis suspect from the out-patient department for screening test 46
(73%) tested positive to leishmaniasis (amastigote) microscopically (Table 1).
Among 46 leishmaniasis
positive clients indicated that 29 (63%) were male and 17 (37%) were female (Table 2).
DISCUSSION
The results findings
based on the clinical signs of the disease and the results of the diagnostic
techniques, has been adequately proven that cutaneous leishmaniasis exist in
north western Nigeria (sokoto State) in high proportion. 63 leishmaniasis
suspect from the out-patient department tested, 46 (73%) tested positive to
leishmaniasis (amastigote) (Table 1 and
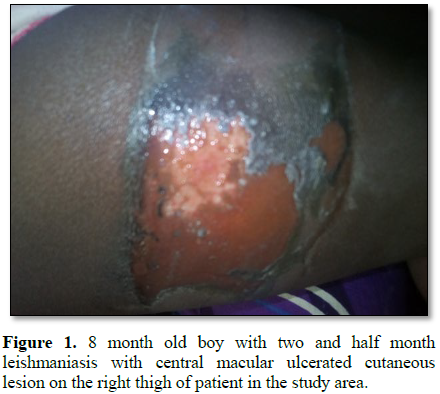
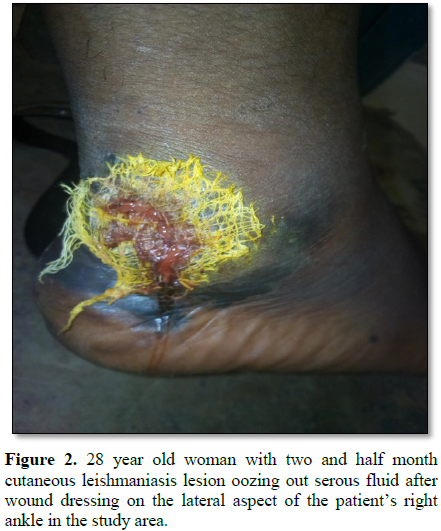
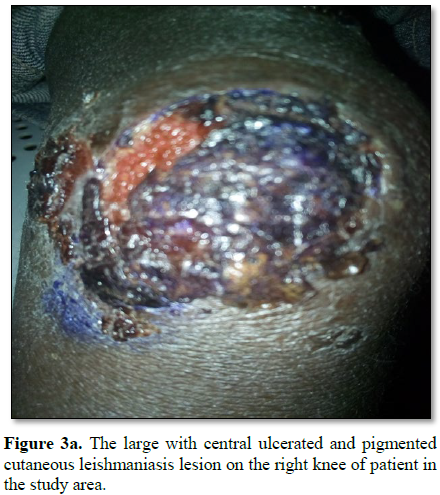
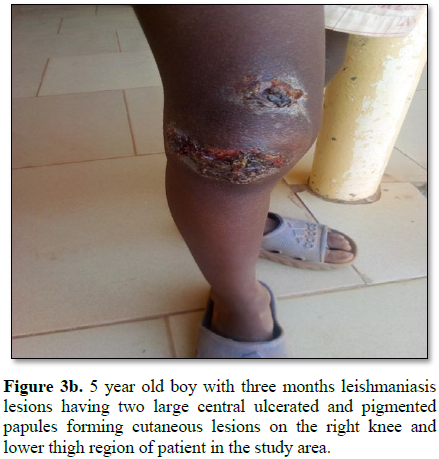
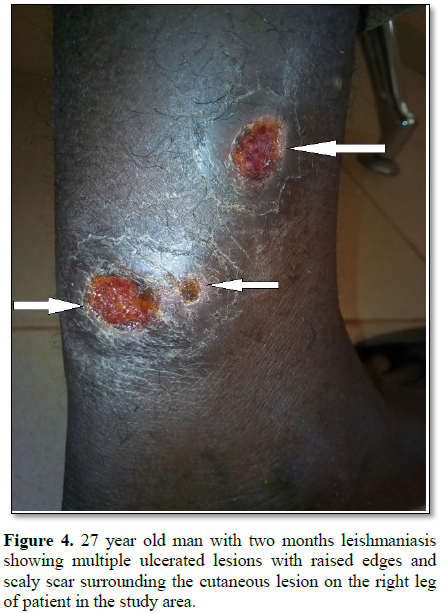
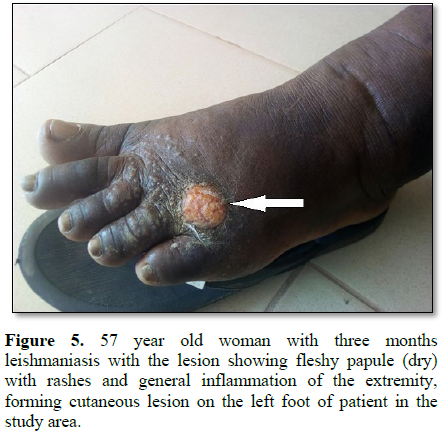
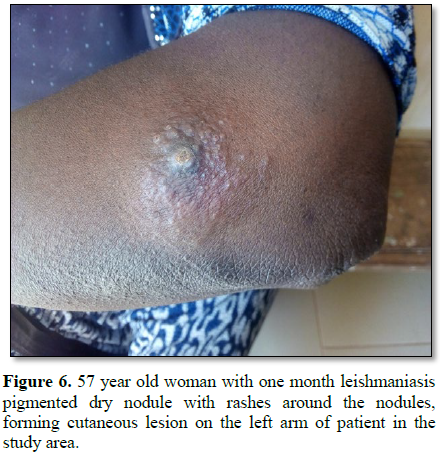
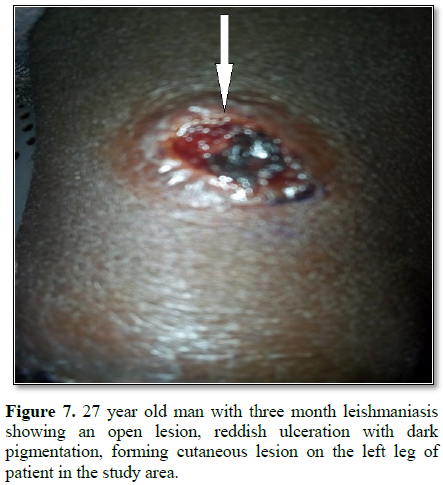
Figures 1-12). 80% of the subject complained they had the bite at night as
they slept outdoor with less covering of the body. This finding confirms the
isolated reports of Obasi [18] and Ishaya et al. [19]. Although the prevalence
rate in this study is contrary to that of Obasi [18] and Ishaya et al. [19]
reporting 6.8%. Mutero et al. [20] reported visceral leishmaniasis to be less
prevalent than malaria, with less than 2% active cases in any age group and had
the same distribution in both sexes in West Pokot district of Kenya study in
1986 where a total of 2,139 people were proportionately screened for the two
diseases according to four age categories (0-4, 5-14, 15-44 and greater than 45
years). The high prevalence observed in this study is because the health centre
is popularly known or a referral site for leishhmaniasis in the state. However,
while the initial sign of cutaneous leishmanasis (CL) found in this work begins
as a tiny, milky-colored papule [21] indicating that it starts as a tiny
reddish papule. The formation of milky-colored papule appearance or and reddish
papule in this study was noticed at different stages of infection and the level
of treatment or how it is managed contrary to the report of Ishaya et al. [19].
Furthermore, the disfiguring dark coloration of the scars pigmentation was
observed in this study. There is general complain of burning, itchiness or the
disgraceful, disfiguring scars on the body surfaces.
In this study it was
noticed that male had higher prevalence (male 29 (63%) than female 17 (37%)) of
this disease, these could be due to high predisposed the male are such as
sleeping in the open space without cover (wear) because of the influence of the
culture and environmental factor such as hot weather when compared to the
females. This confirm the report of Weigel et al. [22] same trend in the
visceral leishmaniasis and cutaneous leishmaniasis research in Ethiopia and
Ecuador, while Ishaya et al. [19] reported that the incidence of CL was higher
in males than in females in Northern Kaduna Nigeria and Northern Jordan
respectively. While Ishaya et al. [19] reported that in southern Kaduna female
had higher prevalence of leishmaniasis (Central (males=5.8%, females=11.0%) and
Southern areas (males=6.0%, females 6.9%)) or about the same as in the males
(Eastern area (males=5.2%; females=5.1%)), where majority of the females are
relatively free from seclusion. Some researchers reported that in Northern
Nigeria, which includes Sokoto State, the males were involved in farm work more
than the females, who were usually secluded on account of Moslem religious
practices.
It was reported that Phlebotomus (Phlebotomus) duboscqui, the transmitting vector of CL
in Nigeria, exists in the Biu, Plateau, Kaduna expectedly too cold for the
vector's survival. These Phlebotomus
(Phlebotomus) duboscqui has migrated
to Sokoto. It was noticed that 95% of the case are from Sokoto central
senatorial area. Another possible reason might be the relatively denser
vegetation and higher humidity in the central senatorial zone of the State,
which might support a correspondingly higher population of reservoir hosts, in
addition to more number of breeding places for the sandflies. Ordinarily, the
disease transmitting vector, P. duboscqui,
naturally survives in severe climatic conditions; these might have a boost of
the presence of the invertebrate vectors, hence the higher point-prevalence in
the region or the senatorial area.
The prevalence rate of
the disease tends to be higher in the age-groups (≥ 15 years) 85% and 15% in ≤
14 year, this is contrary to the report of Ishaya et al. [19] and Agwale et al.
[23] which showed high trend in 1-15 years old, and the 10-15 years old,
respectively, in Keana, Nigeria and Isfahan, Iran respectively. The High
proportion of active lesions in the ages (≥ 15 years) implies higher risk,
predisposing to sand fly bite due to active involvement of the socio-cultural
and economic activity. Ishaya et al. [19] and Agwale et al. [23] could not
observe any direct relationship with age, sex and clinical features of the
cutaneous leishmaniasis lesions in Isfahan, Iran.
CONCLUSION
The prevalence of
leishmaniasis in Sokoto is 46 (73%) tested positive to leishmaniasis out of 63
suspect. Based on the finding of the clinical signs of the disease and the
results of the diagnostic techniques, it has been adequately proven that
cutaneous leishmaniasis exist in Sokoto State in high proportion.
1. Calvopiña
M, Martinez L, Hashiguchi Y (2013) Cutaneous leishmaniasis “chiclero’s ulcer”
in subtropical Ecuador. Am J Trop Med Hyg 89: 195-196.
2. Stowers
JH (1920) Case of Delhi boil or sore (Syn.: Oriental Sore; Aleppo Boil). Proc R
Soc Med 13: 81-83.
3. James
WD, Berger TG (2006) Andrews' diseases of the skin: Clinical dermatology.
Saunders Elsevier, p: 423.
4. Aoun
K, Bouratbine A (2014) Cutaneous leishmaniasis in North Africa: A review.
Parasite 21: 14.
5. The
Institute for International Cooperation in Animal Biologics and the Center for
Food Security and Public Health (2009) Leishmaniasis (cutaneous and visceral)
(PDF). Ames, Iowa: College of Veterinary Medicine, Iowa State University.
6. Vergel
C, Palacios R, Cadena H, Posso CJ, Valderrama L, et al. (2006) Evidence for
leishmania (viannia) parasites in the skin and blood of patients before and
after treatment. J Infect Dis 194: 503-611.
7. Centers
for Disease Control and Prevention (2014) Parasites-leishmaniasis. Resources
for health professionals. Atlanta, Georgia: United States Department of Health
and Human Services.
8. Connolly
MA, World Health Organization (2005) Communicable disease control in
emergencies: A field manual. World Health Organization, p: 152.
9. Banerjee
N (1973) Role of I.M.A. during natural calamities and national emergencies. J
Indian Med Assoc 61: 477-481.
10. Rathi
SK, Pandhi RK, Chopra P, Khanna N (2005) Post-kala-azar dermal leishmaniasis: A
histopathological study. Indian J Dermatol Venereol Leprol 71: 250-253.
11. Singh
N, Ramesh V, Arora VK, Bhatia A, Kubba A, et al. (1998) Nodular post-kala-azar
dermal leishmaniasis: A distinct histopathological entity. J Cutan Pathol 25:
95-99.
12. Stark
D, Pett S, Marriott D, Harkness J (2006) Post-kala-azar dermal leishmaniasis
due to Leishmania infantum in a human immunodeficiency virus type 1-infected
patient. J Clin Microbiol 44: 1178-1180.
13. Du
R, Hotez PJ, Al-Salem WS, Acosta-Serrano A (2016) Old world cutaneous
leishmaniasis and refugee crises in the Middle East and North Africa. PLoS Negl
Trop Dis 10: e0004545.
14. Saroufim
M, Charafeddine K, Issa G, Khalifeh H, Habib RH et al. (2014) Ongoing epidemic
of cutaneous leishmaniasis among Syrian refugees, Lebanon. Emerg Infect Dis 20:
1712-1715.
15. Hiddleston
S (2016) An old disease rears its ugly head. Nature.
16. Sims
A (2016) A disfiguring tropical disease is sweeping across the Middle East. The
Independent.
17. Chotivanich
K, Silamut K, Day NPJ (2006) Laboratory diagnosis of malaria infection - A
short review of methods. Aust J Med Sci 27: 11-15.
18. Obasi
OE (1991) Cutaneous leishmaniasis in Nigeria. Int J Dermatol 30: 274-275.
19. Ishaya
HN, Ibrahim S, Galadima M (2014) Prevalence of cutaneous leishmaniasis in parts
of Kaduna State, Nigeria. Available at: https://www.researchgate.net/publication/237536448
20. Mutero
CM, Mutinga MJ, Ngindu AM, Kenya PR, Amimo FA (1992) Visceral leishmaniasis and
malaria prevalence in West Pokot district, Kenya. East Afr Med J 69: 3-8.
21. Neva
FA, Brown HW (1994) Basic clinical parasitology. (6th Edn). Appleton and Lange:
USA, pp: 57-81.
22. Weigel
MM, Armijos RX, Racines J, Zurita C, Izurieta R, et al. (1994) Cutaneous
leishmaniasis in subtropical Ecuador: Popular perceptions, knowledge and
treatment. Bull Pan Am Health Organ 28: 142-155.
23. Agwale
SM, Duhlinska DD (1995) Leishmanin reactions in school children in Keana,
plateau state, Nigeria. In: 19th Annual Conference Abstracts (A Publication of
the Nigerian Journal of Parasitology), University of Jos, Jos, Nigeria.