751
Views & Citations10
Likes & Shares
INTRODUCTION
Acute and chronic isoniazid (isonicotinic acid hydrazide (INH)) toxicity is associated with HIV subject TB-INH prophylaxis. Acute INH toxicity leads to central nervous system (CNS) toxicity, including seizures, whereas chronic INH toxicity results in hepatotoxicity. The study is aimed at ascertaining the INH hepatotoxicity in HIV subject with TB-INH prophylaxis. Randomized experimental design was employed in this research to determine hepatoxicity and the baseline serum Alanine transaminases (ALT) Aspartate transaminases (AST) level and it level after two months treatment with INH among fifty subjects (50) who voluntary consented to participate. Serum ALT and AST were analysed based on Kinetic determination. Result from this study showed that there is no statistical significant increase ALT mean ± SEM (baseline) before 14.6 ± 6.3 u/l and after INH ingestion 16.4 ± 6.1 u/l since t-test value 0.831 is less than critical value=2.048, AST value shows that there is no statistical significant increase mean ± SEM before 9.6 ± 4.8 u/l and after INH ingestion 12.6 ± 5.3 u/l since t-test value 1.6071 is less than critical value=2.048 in male subjects, among female subject there is statistically significant difference (increase) ALT mean ± SEM (baseline) before 15.3 ± 5.8 u/l and after INH ingestion 19.2 ± 6.2 u/l since t-test value 2.67231 is greater than critical value=1.996 while there is statistically significant increase AST mean ± SEM before 10.4 ± 2.8 u/l and after INH ingestion 14.3 ± 2.7 u/l since t-test value 5.7391 is greater than critical value=1.996. There was statistically significant correlation between serum ALT and AST level before and after two months of INH TB prophylaxis administration in both male and female subject. There were no cases of hepatotoxicity among subject in this study.
INTRODUCTION
Isoniazid, also known as isonicotinyl hydrazide (INH), is an antibiotic used for the treatment of tuberculosis. For active tuberculosis it is often used together with rifampicin, pyrazinamide and either streptomycin or ethambutol. For latent tuberculosis it is often used by itself. It may also be used for atypical types of mycobacteria, such as M. avium, M. kansasii and M. xenopi. It is usually taken by mouth but may be used by injection into a muscle. Isoniazid was first made in 1952. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Common side effects include increased blood levels of liver enzymes and numbness in the hands and feet. Serious side effects may include liver inflammation. It is unclear if use during pregnancy is safe for the baby. Use during breastfeeding is likely okay. Pyridoxine may be given to reduce the risk of side effects [1]. Isoniazid works in part by disrupting the formation of the bacteria's cell wall which results in cell death. Isoniazid is available as a generic medication injection into a muscle. The wholesale cost in the developing world is about 0.60 to 4.75 USD per month [2]. In the United States a month of treatment costs less than 25 USD per month [1]. Isoniazid is approved for latent and active tuberculous infections. For the latter, it must be used in combination with other tuberculosis medications to prevent the development of drug resistance. Isoniazid has been approved as prophylactic therapy for the following populations:
• People with HIV infection and a PPD (purified protein derivative) reaction of at least 5 mm in duration.
• Contacts of people with tuberculosis and who have a PPD reaction at least 5 mm in duration.
• People whose PPD reactions convert from negative to positive in a two year period – at least 10 mm induration for those up to 35 years of age and at least 15 mm induration for those at least 35 years old.
• People with pulmonary damage on their chest X-ray that is likely to be due to healed tuberculosis and also have a PPD reaction at least 5 mm in duration.
• Injection drug users whose HIV status is negative who have a PPD reaction at least 10 mm in duration.
People with a PPD of greater than or equal to 10 mm induration that are foreign-born from high prevalence geographical regions, low-income populations and patients residing in long-term facilities [3].
It is recommended that women with active tuberculosis who are pregnant or breastfeeding take isoniazid. Preventive therapy should be delayed until after giving birth. Nursing mothers excrete a relatively low and non-toxic concentration of INH in breast milk and their babies are at low risk for side effects. Both pregnant women and infants being breastfed by mothers taking INH should take vitamin B6 in its pyridoxine form to minimize the risk of peripheral nerve damage [4]. Vitamin B6 is used to prevent isoniazid-induced B6 deficiency and neuropathy in people with a risk factor, such as pregnancy, lactation, HIV infection, alcoholism, diabetes, kidney failure or malnutrition [5]. People with liver dysfunction are at a higher risk for hepatitis caused by INH and may need a lower dose. Levels of liver enzymes in the bloodstream should be frequently checked in daily alcohol drinkers, pregnant women, IV drug users, people over 35 and those who have chronic liver disease, severe kidney dysfunction, peripheral neuropathy or HIV infection since they are more likely to develop hepatitis from INH [6]. Up to 20% of people taking isoniazid experience peripheral neuropathy when taking doses of 6 mg/kg of weight daily or higher [7]. Gastrointestinal reactions include nausea and vomiting. Aplastic anemia, thrombocytopenia and agranulocytosis due to lack of production of red blood cells, platelets, and white blood cells by the bone marrow respectively, can also occur. Hypersensitivity reactions are also common and can present with a maculopapular rash and fever. Acute INH toxicity leads to central nervous system (CNS) toxicity, including seizures, whereas chronic INH toxicity results in hepatotoxicity. Since 1952, INH has been used as a front-line antimicrobial for tuberculosis (TB) [8]. INH is commonly used for prophylaxis of patients with a recently converted Mantoux tuberculin skin test (TST) with purified protein derivative (PPD) or in conjunction with other medications for the treatment of active TB infection. A typical regimen for tuberculosis includes INH, rifampin, pyrazinamide and ethambutol or streptomycin. Treatment lasts for 6 months for active TB, assuming responsiveness to antimicrobial therapy. Although the exact mechanism of activity is unknown, INH is believed to act by interfering with mycobacterial cell wall synthesis. People taking isoniazid and acetaminophen are at risk of acetaminophen toxicity. Isoniazid is thought to induce a liver enzyme which causes a larger amount of acetaminophen to be metabolized to a toxic form [9]. Isoniazid decreases the metabolism of carbamazepine, thus slowing down its clearance from the body. People taking carbamazepine should have their carbamazepine levels monitored and, if necessary, have their dose adjusted accordingly [10]. It is possible that isoniazid may decrease the serum levels of ketoconazole after long term treatment. This is seen with the simultaneous use of rifampin, isoniazid and ketoconazole [11]. Isoniazid may increase the amount of phenytoin in the body. The doses of phenytoin may need to be adjusted when given with isoniazid [12,13]. Isoniazid may increase the plasma levels of theophylline. There are some cases of theophylline slowing down isoniazid elimination. Both theophylline and isoniazid levels should be monitored [14].
METABOLISM AND MECHANISM OF ACTION
The most likely biochemical mechanism is that the metabolism of INH produces reactive metabolites that bind and damage cellular macromolecules in the liver. The presumed mechanism of INH-induced seizure involves a decrease in the availability of GABA, which is the major inhibitory neurotransmitter in the central nervous system (CNS), as well as a relative increase in the amounts of glutamate, the primary excitatory neurotransmitter. INH metabolites directly inhibit pyridoxine phosphokinase. This enzyme converts pyridoxine (vitamin B-6) to its active form, pyridoxal-5'-phosphate, a key cofactor in the production of GABA. This functional depletion of pyridoxine causes a disruption of glutamate and GABA homeostasis and leads to an excessive excitatory milieu in the brain. INH is mostly acetylated via the liver and the subsequent product, acetylisoniazid, and then is further (1) eliminated by the kidney; (2) oxidized to hydroxylamine, a toxic metabolite; (3) hydrolyzed into hydrazine, also toxic; or (4) further hydrolyzed to another toxic compound, acetylhydrazine. Patients who are slow acetylators may be at higher risk for hepatotoxicity [15,16]. The mild elevation of transaminases seen in as many as 20% of patients who are treated during the first 2 months of therapy may reflect direct toxicity from hydrazine metabolites, which can covalently bind to cellular macromolecules, including DNA. The more severe form of hepatitis, seen in up to 1% of adults who are treated, may be a consequence of the production of more reactive species by the CYP-450 enzyme system [17]. Although the most common presentation of INH hepatotoxicity is hepatocellular damage, patients occasionally may present with true drug hypersensitivity characterized by skin rash, fever, and eosinophilia [16]. Isoniazid works in part by disrupting the formation of the bacteria's cell wall [18]. Isoniazid is a prodrug and must be activated by a bacterial catalase-peroxidase enzyme in Mycobacterium tuberculosis called KatG [19]. KatG couples the isonicotinic acyl with NADH to form isonicotinic acyl-NADH complex. This complex binds tightly to the enoyl-acyl carrier protein reductase known as InhA, thereby blocking the natural enoyl-AcpM substrate and the action of fatty acid synthase. This process inhibits the synthesis of mycolic acids, which are required components of the mycobacterial cell wall. A range of radicals are produced by KatG activation of isoniazid, including nitric oxide [20], which has also been shown to be important in the action of another anti-mycobacterial prodrug pretomanid [21]. Isoniazid is bactericidal to rapidly dividing mycobacteria, but is bacteriostatic if the mycobacteria are slow-growing [22].
STATEMENT OF PROBLEM
US Public Health Service (USPHS) in 1978 found that about 10% of patients with mild transaminase elevations (1-2% of all adults treated) progress to severe hepatitis and liver failure unless the drug is discontinued [23]. Death occurred in 8 patients (0.06%). When INH is given together with other drugs for active TB, the incidence of severe hepatotoxicity is greater. In a review of possible INH-associated hepatitis fatalities identified between 1969 and 1989, a total of 38% occurred in black patients, 40% in non-Hispanic white patients, 15% in Hispanics, 4% in Native Americans and 1% in Asians [23]. There is high incidence and risk of hepatoxicity induced by INH as prophylaxis against TB, hence monitoring the liver enzyme has become imperative. The above reason prompted the research on the hepatotoxicity induced by INH prophylaxis among HIV subject.
OBJECTIVE OF THE STUDY
Determine prevalence of INH hepatoxicity in HIV subject on HAART.
Specific objectives
i. Determine the baseline of serum liver transaminases among HIV subject on HAART.
ii. Determine the serum liver transaminases level after INH prophylaxis in HIV subject on HAART.
iii. Determine the relationship of serum liver transaminases level baseline and after INH prophylaxis in HIV subject on HAART.
SIGNIFICANCE OF THE STUDY
The study will provide knowledge of Baseline for accessing INH toxicity for health workers, monitor and prevent the complication that might arise from hepatoxicity of INH, which could reduce morbidity and mortality on INH toxicity.
RESEARCH QUESTIONS
The following research questions gave direction to the study.
1. What is prevalence of INH hepatoxicity in among the clients in the facility?
2. What is the influence of gender (male and female) on the level of AST and ALT resulting from INH prophylaxis therapy?
3. What is the serum AST and ALT trend across the client’s baseline and after INH prophylaxis?
HYPOTHESES
The following null hypotheses were postulated for the study:
1. There is no prevalence of INH hepatoxicity in among the clients in the facility.
2. There is no significant difference in the level serum ALT and AST among male and female clients after INH prophylaxis.
3. There is no significant difference increases in the serum AST and ALT across the clients baseline and after INH prophylaxis.
SCOPE OF THE STUDY
The study was delimited to HIV subjects at the age (18-70 years) receiving care (HAART) at the facility. It was delimited to independent variables to be assayed which are ALT and AST only.
RESEARCH DESIGN
Randomized experimental design was employed in this research to determine hepatoxicity and the baseline transaminases level and it level after treatment with INH among subject who voluntary consented to participate.
Area of the study
Sokoto is one of the seven states that form the North West geopolitical zone of Nigeria. It is bordered to the north by the Republic of Niger, Zamfara State to the east, Kebbi state to the south and west. It is situated in the savannah on the temperature of 44 degree Celsius annually. The city of Sokoto is its capital. Sokoto state traces its origin to the Sokoto Caliphate founded in 1809 by ShehuUsmandanFodio, the leader of the jihadists who overthrew the Hausa state of Gobir, Kano, Katsina and Kanem-Bornu. The empire fell after the British conquest of 1903 and the death of Attahiru, the Sultan of Sokoto and became part of the Northern Region in the three-region structure of 1954. In 1967, Nigeria, the military administration of General Yakubu Gowon merged Sokoto and Niger provinces to form the North Western state. In 1976, North Western State was spilt into Sokoto and Niger states by the military administration of General Murtala Muhammed. Sokoto State covers an area of 28,232.37 km2. The state is located between latitudes 40 to 60 north and longitudes 110 to 130 east has a population of 3,702,676 (2006 census figures). It accounts for 2.3% of Nigeria’s total population. Prior to the establishment of Sokoto as a ribat (military camp or frontier) in 1809, the area that is modern-day Sokoto state was home to Hausa state with large populations. These states eventually fell under the control of Usmanudan Fodio and the Fulani jihadists and became part of the Sokoto Caliphate. In 1817 when Usman died, his son Muhammed Bello succeeded him as the Sultan of Sokoto. Usman’s brother Abdullahi was given the western divisions of the caliphate to run; however, supreme authority rested with Bello. At the height of its power, the Sokoto Caliphate extended as far as Ilorin (in modern-day Kwara State). The Hausa are the largest ethnic group in Sokoto State while the Fulani are its second largest. Minority include the Zabarmawa, Tuareg and the Dakarkari. The majority of the population is Sunni Muslim. There is a small Shia minority. There are twenty-three local government areas (LGAs) in Sokoto. Each has a chairman as its administrative head. The Islamic community in Nigeria considers the person of the Sultan as ‘First among Equals’. He is both the political head of the Fulani as well as the supreme spiritual head of the rough 70 million Muslims in Nigeria. Currently occupying the site is Sultan MuhammaduSa’ad Abubakar III, the twentieth sultan of Sokoto. Agriculture is the mainstay of Sokoto’s economy. The riverine floodplains produce cash crops, including peanuts (groundnuts), cotton and rice. Sorghum, millet, cowpeas and cassava are grown in the upland areas. Much of the land in the state is used for grazing cattle. Cattle hides, goatskin, sheepskins and finished leather products are significant exports, as are cattle, goats and fowl. The state possesses limestone and kaolin deposits and Sokoto City, the state capital, is home to a cement factory, tanneries and a modern abattoir. Festivals include Kalankuwa, Halbi, Sharo, Aikin Gawa, Shan Gumba-Pap drinking and Remo Fishing Festival. The stress associated with agriculture could increase the incidence of hypertension. The trend of cigarette smoking is very high among adult in Sokoto metropolis.
ETHICAL APPROVAL
Ethical clearance was obtained from the Ethical Committee of the 1 Brigade Medical Centre, Ginginya barrack, Sokoto and seeks permission for collection data. This study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the ethical research committee of the 1 Brigade Medical Centre, Ginginya barrack, Sokoto.
TARGET POPULATION
The target population was all the HAART clients receiving care at clinic in 1 Brigade Medical Centre Sokoto. The sample size was calculated using the formula:
Where,
n=sample size
p=Prevalence of HIV in
Nigeria=3.2
q=100 – p
=100 – 4.7
=96.8
E=allowable error=5%
N=3.2 × 96.8 /
[5/1.96]2
=309.76 / 6.51
=47.6
=48
=48+4 (10% for attrition)
=52 approximately patients.
Sample size/sampling technique
Fifty (50) Subjects were randomly selected from among the population, blood sample were collected two times, first (baseline) the day before client starts INH prophylaxis, second two months after taking INH prophylaxis.
Inclusion criteria
(i) All adult HIV seropositive patients, who consent to be enrolled into the study.
(ii) All adult HIV seropositive patients, without any sign and symptom of liver disease.
(iii) All adult HIV seropositive, without of any underlying disease like epilepsy, seizure or hypersensivity reaction like skin rash.
(iv) All adult HIV seropositive most have been on HAART.
Exclusion criteria
(i) All adult HIV seropositive patients who did not consent to be enrolled into the study.
(ii) All adult HIV seropositive patients with sign and symptom of liver disease.
(iii) All adult HIV seropositive patients with underlying disease like epilepsy, seizure or hypersensivity reaction like skin rash.
(iv) All adult HIV seropositive not on HAART or naïve.
COLLECTION OF SAMPLES
Ten (6) ml of blood samples were collected in plain bottle. This was allowed to clot within 30 min of collection. The samples were then centrifuged at 3000 rpm for 5 min to obtain neat serum samples, which were harvested into bijou bottles and labeled accordingly.
Analytical methods
AST analysis [24]:
Principle: Kinetic determination of AST based upon the following reaction.
AST
L-Asparate + alpha-ketoglutarate ——› Oxaloacetate + L-Glutamate
MDH
Oxaloacetate + NADH + +H+ ——› L-Malate + NAD+
AST: Aspartate Aminotransferase
MDH: Malate Dehydrogenase
R1: Tris buffer (pH 7.8) 88 mmol/l; L-Aspartate 260 mmol/l; MDH>900 U/L; LDH>1500 U/L
R2: Alpha-ketoglutarate 12 mmol/l; NADH 0.24 mmol/l
Reagent preparation: Mix 4 volume of reagent 1 (R1) with 1 volume of reagent 2 (R2). This working reagent is stable for 30 days at 2-8°C.
Procedure of semi auto analyzer: Add 1000 µl of working reagent, followed by 100 µl of sample into a cuvette mix and incubate at 37°C for 1 min. measure the change in absorbance per 20 s (OD/min) during 1 min.
Calculation:
SGOT activity (U/L) = (OD/min) × 1745
Reference range: Upto 46 U/L
ALT analysis [24]:
Principle: Kinetic determination of ALT based upon the following reaction.
ALT
L-Alanine + alpha-ketoglutarate ——› pyruvate + L-Glutamate
LDH
Oxaloacetate + NADH + +H+ ——› L-Lactate + NAD+
ALT: Aspartate Aminotransferase
LDH: Malate Dehydrogenase
R1: Tris buffer (pH 7.5) 110 mmol/l; L-Alanine 600 mmol/l; LDH>1500 U/L
R2: Alpha-ketoglutarate 16 mmol/l; NADH 0.24 mmol/l
Reagent preparation: Mix 4 volume of reagent 1 (R1) with 1 volume of reagent 2 (R2). This working reagent is stable for 30 days at 2-8°C.
Procedure of semi auto analyzer: Add 1000 µl of working reagent, followed by 100 µl of sample into a cuvette. Mix and incubate at 37°C for 1 min. Measure the change in absorbance per 20 s (OD/min) during 1 min.
Calculation:
SGOT activity (U/L) = (OD/min) × 1745
Reference range: Upto 49 U/L
Method of data analysis
Data collected were analyzed using descriptive statistic of grand mean and standard error mean, correlation as well as inferential statistics of t-test. The level of significant was fixed at 0.05. Appropriate degrees of freedom were worked out.
RESULTS
Table 1 shows that there is no statistical significant increase ALT mean ± SEM before 14.6 ± 6.3 u/l and after INH ingestion 16.4 ± 6.1u/l since t-test value 0.831is less than critical value=2.048, AST value shows that there is no statistical significant increase mean ± SEM before 9.6 ± 4.8 u/l and after INH ingestion 12.6 ± 5.3 u/l since t-test value 1.6071 is less than critical value=2.048 in male subject, among female subject there is statistically significant difference (increase) ALT mean ± SEM before 15.3 ± 5.8 u/l and after INH ingestion 19.2 ± 6.2 u/l since t-test value 2.67231 is greater than critical value=1.996 while there is statistically significant increase AST mean ± SEM before 10.4 ± 2.8 u/l and after INH ingestion 14.3 ± 2.7 u/l since t-test value 5.7391 is greater than critical value=1.996.
DISCUSSION
TB is a leading killer of people with HIV and in resource-limited settings many people with HIV who start ART late, after they have already developed advanced immune suppression, die within the first six months on treatment, often due to TB [25]. INH hepatotoxicity is a common complication of anti-tuberculosis therapy, ranging in severity from asymptomatic elevation of serum transaminases to hepatic failure necessitating liver transplantation. This toxicity is not caused by high plasma INH levels but appears to represent an idiosyncratic response. This study showed that there is no statistical significant increase ALT mean ± SEM baseline 14.6 ± 6.3 u/l and after INH ingestion 16.4 ± 6.1 u/l since t-test value 0.831 is less than critical value=2.048, AST value shows that there is no statistical significant increase mean ± SEM baseline 9.6 ± 4.8 u/l and after INH ingestion 12.6 ± 5.3 u/l since t-test value 1.6071 is less than critical value=2.048 in male subject, among female subject there is statistically significant difference (increase) ALT mean ± SEM baseline 15.3 ± 5.8 u/l and after INH ingestion 19.2 ± 6.2 u/l since t-test value 2.67231 is greater than critical value=1.996 while there is statistically significant increase AST mean ± SEM baseline 10.4 ± 2.8 u/l and after INH ingestion 14.3 ± 2.7 u/l since t-test value 5.7391 is greater than critical value=1.996. INH hepatotoxicity was not observed among subject contrary to the report of Burk et al. [26] and Chamorro et al. [27]. Vitamin B6 (pyridoxine) supplement to prevent isoniazid-induced B6 deficiency and neuropathy in people with a risk factor, such as pregnancy, lactation, HIV infection, alcoholism, diabetes, kidney failure or malnutrition and to reduce or eliminate INH hepatotoxicity which is characterized by Symptoms typically precede jaundice and liver failure by only a few days. Constitutional symptoms include fatigue, anorexia, nausea, myalgia, and arthralgia. Symptoms due to liver failure include jaundice, dark urine, light-colored stools, bleeding diathesis, pruritus, confusion and coma. Symptoms due to hepatic inflammation include right upper quadrant tenderness and gastrointestinal (GI) distress including anorexia, nausea and vomiting, the subject in this study never had pyridoxine supplement still no case of toxicity was recorded. The increase in serum transaminases was clinically not significant because they fall between the reference ranges. Asymptomatic elevation of serum liver enzyme concentrations occurs in 10% to 20% of people taking INH, and liver enzyme concentrations usually return to normal even when treatment is continued reported by CDC [28]. Isoniazid-induced liver toxicity has been shown to occur in 50% of patients within the first 2 months of therapy [28].
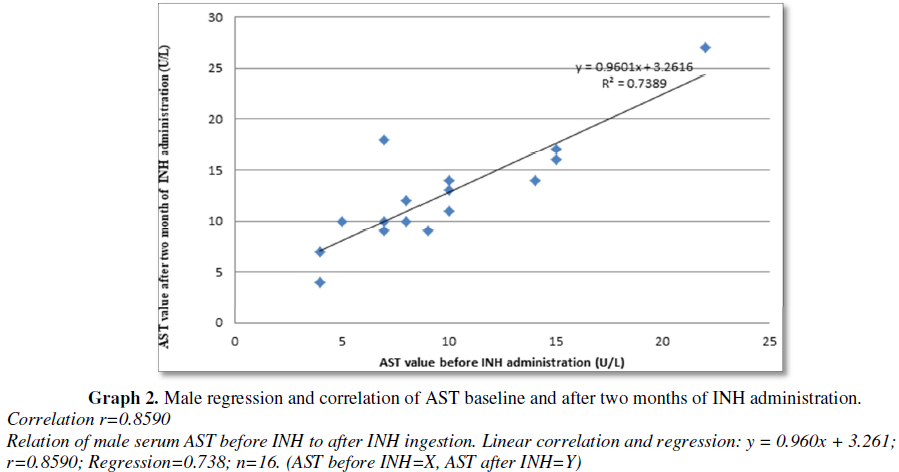
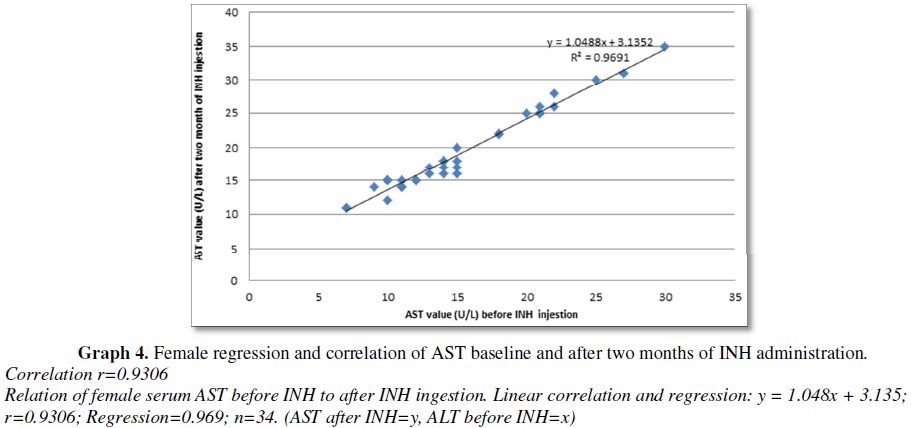
There was statistically significant correlation between serum ALT and AST level before and after two months of INH TB prophylaxis administration in both male and female subject. Among male subject there were strong correlation and regression: y = 0.945x + 2.607; r=0.9808; Regression=0.962; n=16. (ALT before INH=y, ALT after INH=x) (Graph 1), there were statistically significant increase in ALT after INH administration and strong correlation and regression: y = 0.960x + 3.261; r=0.8590; Regression=0.738; n=16 (AST before INH=X, AST after INH=Y), there was statistically significant increase in AST after INH administration (Graph 2). Among female subject there was strong correlation and regression: y = 1.048x + 3.135; correlation (r)=0.9844; Regression=0.969; n=34. (ALT after INH ingestion=y, ALT before INH=x) (Graph 3), there were statistically significant increase in ALT after INH administration and strong correlation and regression: y = 1.048x + 3.135; correlation (r)=0.9306; Regression=0.969; n=34. (AST after INH=y, AST before INH=x), there were statistically significant increase in AST after INH administration (Graph 4). Fernandez-Villar et al. [29] reported that 34 patients (8.2%), had elevations in the ALT and/or AST values to >3 times ULN were detected. None of these patients met the hepatotoxicity criteria or had symptoms of hepatitis, and the transaminase values returned to baseline values even though isoniazid therapy was maintained. Eleven patients (32.3%) were heavy drinkers. Twenty (4.8%) of 415 patients (95% CI, 37.4) developed isoniazid-associated hepatotoxicity and isoniazid was withdrawn. The ALT, AST and bilirubin peak values reached in these subjects were 339 ± 201 IU/L (range, 207948 IU/L), 184 ± 114 IU/L (range, 73482 IU/L) and 0.8 ± 0.3 mg/dL (range, 0.11.6 mg/dL), respectively. Nineteen (95%) of these 20 patients were men, with a mean age of 30.3 ± 6.6 years. Only 6 patients (1.4%; 95% CI, 0.53.2) had symptoms of hepatitis, such as asthenia, nausea, or mild abdominal pain. None of them presented with jaundice or other manifestations that would imply serious hepatitis. The time until the diagnosis of toxicity was 61.3 ± 28 days (range, 21120 days). In 17 (85%) of 20 patients, the diagnosis was made in the first 3 months, and in only 3 patients was the diagnosis made thereafter (at the end of the fourth month).
CONCLUSION
There was no record of hepatoxicity among HIV subject that had INH prophylaxis to eliminate the risk of TB infection. Although there was strong statistically significant correlation of serum ALT and AST baseline and after INH administration.
1. Hamilton
R (2015) Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones
& Bartlett Learning, p: 49.
2. (2008)
International Drug Price Indicator Guide. Isoniazid.
3. Recommendations
of the Advisory Committee for Elimination of Tuberculosis (1990) The use of
preventive therapy for tuberculosis infection in the United States. Morbid
Mortal Wkly Rep MMWR 39: 9-12.
4. Bothamley
G (2001) Drug treatment for tuberculosis during pregnancy: Safety
considerations. Drug Saf 24: 553-565.
5. Steichen
O, Martinez-Almoyna L, De Broucker T (2006) Isoniazid induced neuropathy:
Consider prevention. Rev Mal Respir 23: 157-160.
6. Saukkonen
JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, et al. (2006) An official ATS
statement: Hepatotoxicity of anti-tuberculosis therapy. Am J Respir Crit Care
Med 174: 935-952.
7. Alldredge
B (2013) Applied therapeutics. Available at: https://www.drugs.com/
8. Agrawal
RL, Dwivedi NC, Agrawal M, Jain S, Agrawal A (2008) Accidental isoniazid
poisoning - A report. Indian J Tuberc 55: 94-96.
9. Murphy
R, Swartz R, Watkins PB (1990) Severe acetaminophen toxicity in a patient
receiving isoniazid. Ann Intern Med 113: 799-800.
10. Fleenor
ME, Harden JW, Curtis G (1991) Interaction between carbamazepine and
anti-tuberculosis agents. Chest 99: 1554.
11. Baciewicz
AM, Baciewicz FA Jr (1993) Ketoconazole and fluconazole drug interactions. Arch
Intern Med 153: 1970-1976.
12. Jonville
AP, Gauchez AS, Autret E, Billard C, Barbier P, et al. (1991) Interaction
between isoniazid and valproate: A case of valproate over dosage. Eur J Clin
Pharmacol 40: 197-198.
13. Bass
JB Jr, Farer LS, Hopewell PC, O'Brien R, Jacobs RF, et al. (1994) Treatment of
tuberculosis and tuberculosis infection in adults and children. Am J Respir
Crit Care Med 149: 1359-1374.
14. Höglund
P, Nilsson LG, Paulsen O (1987) Interaction between isoniazid and theophylline.
Eur J Respir Dis 70: 110-116.
15. Ben
Mahmoud L, Ghozzi H, Kamoun A, Hakim A, Hachicha H, et al. (2012) Polymorphism
of the N-acetyl transferase 2 gene as a susceptibility risk factor for
anti-tuberculosis drug-induced hepatotoxicity in Tunisian patients with
tuberculosis. Pathol Biol (Paris) 60: 324-330.
16. Santos
NP, Callegari-Jacques SM, Ribeiro Dos Santos AK, Silva CA, et al. (2013)
N-acetyl transferase 2 and cytochrome P450 2E1 genes and isoniazid-induced
hepatotoxicity in Brazilian patients. Int J Tuberc Lung Dis 17: 499-504.
17. Roy
PD, Majumder M, Roy B (2008) Pharmacogenomics of anti-TB drugs-related
hepatotoxicity. Pharmacogenomics 9: 311-321.
18. (2008)
The American Society of Health-System Pharmacists. Isoniazid.
19. Suarez
J, Ranguelova K, Jarzecki AA, Manzerova J, Krymov V, et al. (2009) An oxyferrousheme/protein-based
radical intermediate is catalytically competent in the catalase reaction of
Mycobacterium tuberculosis catalase-peroxidase (KatG). J Biol Chem 284:
7017-7029.
20. Suarez
J, Ranguelova K, Jarzecki AA, Manzerova J, Krymov V, et al. (2009) An
oxyferrousheme/protein-based radical intermediate is catalytically competent in
the catalase reaction of Mycobacterium tuberculosis catalase-peroxidase (KatG).
J Biol Chem 284: 7017-7029.
21. Singh
R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, et al. (2008) PA-824
kills non-replicating Mycobacterium tuberculosis by intracellular NO release.
Science 322: 1392-1395.
22. Ahmad
Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, et al. (2009) Biphasic kill
curve of isoniazid reveals the presence of drug‐tolerant, not drug‐resistant,
Mycobacterium tuberculosis in the Guinea Pig. J Infect Dis 200: 1136-1143.
23. Kopanoff
DE, Snider DE Jr, Caras GJ (1978) Isoniazid-related hepatitis: A US Public
Health Service cooperative surveillance study. Am Rev Respir Dis 117: 991-1001.
24. Thefeld
W, Hoffmeister H, Busch EW, Koller PU, Vollmar J (1994) Dtsch Med Wochenschr
99: 343-351.
25. Hosseinpour
MC, Bisson GP, Miyahara S, Sun X, Moses A (2016) Empirical tuberculosis therapy
versus isoniazid in adult outpatients with advanced HIV initiating
antiretroviral therapy (REMEMBER): A multi-country open-label randomised
controlled trial. Lancet 387: 1198-1209.
26. Burk
RF, Hill KE, Hunt RW Jr, Martin AE (1990) Isoniazid potentiation of
acetaminophen hepatotoxicity in the rat and 4-methylpyrazole inhibition of it.
Res Commun Chem Pathol Pharmacol 69: 115-118.
27. Chamorro
JG, Castagnino JP, Musella RM, Nogueras M, Aranda FM (2013) Sex, ethnicity and
slow acetylator profile are the major causes of hepatotoxicity induced by
anti-tuberculosis drugs. J Gastroenterol Hepatol 28: 323-238.