959
Views & Citations10
Likes & Shares
We
investigated the effect of the hydroxylation of benzoic and cinnamic acids and
the effect of cyanating of cinnamic acid at a-position on their uptake by
Caco-2 cells via monocarboxylic acid transporters (MCTs). Benzoic and cinnamic
acids are typical substrates for MCTs. The mono-hydroxylation of benzoic acid
at the m- and p-positions markedly decreased uptake, whereas that at the o-position decreased uptake only
marginally, probably due to the decrease in lipophilicity and the formation of
internal hydrogen bonds between the carboxyl and hydroxyl groups, respectively.
The di-hydroxylation of benzoic acid decreased uptake drastically, and the
substitution of the hydroxyl group in the benzoic acid with a methoxy group
increased uptake, probably due to the respective decrease and increase in
lipophilicity. Similarly, mono-hydroxylation of cinnamic acid led to a marked
decrease in uptake, which was decreased even further by di-hydroxylation. The
uptake of cinnamic acid derivatives such as a-cyanocinnamic acid, a-cyano-4-hyrdoxycinnamic
acid and a-cyano-3-hydroxycinnamic acid was markedly
lower than that of cinnamic acid, and the uptake of a-methylcinnamic acid fell between
that of cinnamic acid and the a-cyanocinnamic acid derivative.
The uptake of benzoic acid was markedly decreased by coincubation with most
benzoic and cinnamic acid derivatives, and the cinnamic acids competitively
decreased the uptake of benzoic acid. Thus, the hydroxylation of benzoic and
cinnamic acids and the cyanating of cinnamic acids at a position could result in decrease
of their uptake by Caco-2 cell via MCTs.
Keywords: Benzoic acid, Cinnamic acid, Phenoxyacetic
acid, Monocarboxylic acid transporters (MCTs), Caco-2 cells.
INTRODUCTION
Monocarboxylic
acid transporters (MCTs) are members of the SLC16A gene family, and consist of
14 members, of which only the first four (MCT1-MCT4) are characterized as
proton-linked monocarboxylic acid transporters [1,2]. MCTs have an important
role in the intestinal absorption of not only short-chain monocarboxylic acids
such as lactic acid, propionic acid and pyruvic acid, but also exogenous
monocarboxylic acid compounds such as benzoic acid, phenolic acid, and
pharmacologically active compounds [3-9].
The human colorectal adenocarcinomal cell line Caco-2 is a useful model with which to study the intestinal absorption of various compounds, since the cells are morphologically and functionally similar to human small intestinal epithelial cells [10]. Caco-2 cells express various MCT subtypes, such as MCT1, MCT3, MCT4, MCT5 and MCT6, with MCT1 being the most abundant [11]. a-Cyanocinnamate analogs are known to inhibit MCT1, MCT2 and MCT4 activities, and a-cyano-4-hydroxycinnamic acid (CHC) is used as an inhibitor of those MCTs [1,12]. Benzoic acid and some of its derivatives, fluorescein, and cinnamic acid and some of its derivatives are typical substrates for MCTs and have been used as competitive inhibitors in several studies on MCTs [3,7,9,13,14], although the MCT subtype that mediated their transport has not been elucidated.
We investigated the uptake mechanisms of phenoxyacetic acid
derivatives, such as 4-chlorophenoxyacetic acid (4-CPA),
4-chloro-2-methylphenoxyacetic acid (MCPA), 2,4-dichlorophenoxyacetic acid
(2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), and suggested that the
uptake of those derivatives from the apical membranes of Caco-2 cells is
mediated via the same MCTs as benzoic acid, at least in part, and the increase
in lipophilicity due to the chloro-substituent may increase the uptake via the
MCTs [15,16]. Furthermore, the uptake of 3,5,6-trichloro-2-pyridinyloxyacetic
acid (triclopyr) from the apical membranes of Caco-2 cells appears to occur via
MCTs, whereas the uptake of 3,6-dichloro-2-methoxybenzoic acid (dicamba) mainly occurs via
passive diffusion, as coincubation with benzoic acid markedly decreased the
uptake of triclopyr, in the same manner as the phenoxyacetic acid derivatives,
while coincubation with benzoic acid did not decrease the uptake dicamba, and
the uptake of dicamba was markedly lower than triclopyr [17]. On the other
hand, coincubation with CHC sparingly decreased the uptake of phenoxyacetic
acid derivatives [15,16], dicamba [17] and benzoic acid (our unpublished data).
We recently investigated the structural effect of benzoic acid on its
uptake into Caco-2 cells via MCTs, with a particular focus on chloro- and
methoxy-substituted benzoic acid, and reported that most of the chloro- and
methoxy-substitutions at the benzene ring did not so affect initial uptake, but
2,6-disubstitution markedly decreased uptake, probably due to the inhibitory
effect of 2,6-disubstitution on the accessibility of carboxylic group to MCTs
located on the apical membranes of Caco-2 cells [18]. On the other hand,
Konishi et al. [7] investigated the structural effects of benzoic and cinnamic
acid derivatives on the transport of fluorescein through Caco-2 cell
monolayers, and reported that the most of the derivatives mono-hydroxylated at
the benzene ring had an inhibitory effect on fluorescein transport via MCTs,
but the di- and tri-hydroxylated derivatives did not.
In the present study, we investigated the structural effect of
hydroxylation at the benzene ring of benzoic, cinnamic and phenoxyacetic acids
on their uptake into Caco-2 cells via MCTs, and compared our results with the
previous results for chloro-substitution [18]. Furthermore, we investigated the
inhibitory effects of the a-cyano group of cinnamic acids on
MCT activity.
MATERIALS
AND METHODS
Materials
Benzoic acid, 2-,3-,4-hydroxybenzoic acids, 2,4-, 2,6- and
3,4-dihydroxybenzoic acids, 2-,3-, 4-methoxybenzoic acids,
2-hydroxy-3-methoxybenzoic acid, 3,4-dimethoxybenzoic acid, 3-hydroxy-4-methoxybenzoic
acid, 4-hydroxy-3-methoxybenzoic acid, a-cyano-3-hydroxycinnamic acid,
3-hydroxycinnamic acid, trans-cinnamic
acid, 3,4-dihydroxycinnamic acid, 3-hydroxy-4-methoxycinnamic acid,
phenoxyacetic acid, 4-hydroxyphenoxyacetic acid, 2,4-dichlorophenoxyacetic acid
(2,4-D) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Wako
Pure Chemical Industries, Ltd. (Osaka, Japan). a-Cyanocinnamic acid and a-methylcinnamic
acid were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan).
2,3-Dihydroxybenzoic acid, 4-hydroxycinnamic acid, 4-hydroxy-3-methoxycinnamic
acid and a-cyano-4-hydroxycinnamic acid were purchased
from Sigma-Aldrich Co. (St. Louis, MO). Fetal bovine serum (FBS) and
nonessential amino acid (NEAA) were obtained from Life Technologies (Carlsbad,
CA). All other chemicals used were of the highest purity commercially
available.
Cell culture
Caco-2 cells at passage 46 were obtained from RIKEN Cell Bank (Tsukuba,
Japan), and cells between passage 60 and 85 were used in this study. As
described previously [19], the cells were cultured on 35-mm six-well culture
dishes coated with rat collagen type I (Becton Dickinson, Bedford, MA) in DMEM
containing FBS (10%), NEAA (1%), streptomycin (100 µg/mL) and penicillin G (70
U/mL) at 37°C in a humidified atmosphere of 5% CO2-95% air. The
culture medium was replaced three times a week, and the confluent Caco-2 cell
monolayers, cultured for 10-14 days, were used in this study.
Uptake study using
Caco-2 cells
The uptake experiment was performed as described previously [18,19].
The culture medium was removed, and the cells were preincubated at 37°C for 20
min in 1.5 mL of the incubation medium at pH 7.4. After preincubation, the
medium was aspirated, and the cells were incubated with 1.5 mL of fresh
incubation medium, at pH 6.0, containing benzoic acid with or without the
benzoic or cinnamic acid derivatives for 1 min at 37°C. The incubation medium
used for the uptake study was Hanks’ balanced salt solution containing 25 mM
D-glucose and 10 mM MES at pH 6.0 or 10 mM HEPES (pH 7.4).
After the incubation with benzoic or cinnamic acid derivatives, the
cell surface was quickly washed three times with ice-cold incubation medium.
The cells were suspended in 1.0 mL of extraction solution (1 N H3PO4:
methanol = 1:1) for 60 min at room temperature, subsequently scraped off, and
collected using a cell scraper [18,19]. The suspension was centrifuged at
13,000g for 10 min, and a 100-µL
aliquot of the supernatant was injected onto the HPLC system.
Determination of
benzoic acid derivatives, cinnamic acid derivatives and protein
Benzoic and cinnamic acid derivatives were determined using HPLC system
consisting of a Shimadzu LC-10A pump and SPD-10A UV detector (Kyoto, Japan).
The column used was an Inertsil VP-ODS (4mm i.d. x 250 mm; GL Sciences, Tokyo,
Japan), the flow rate was 1.0 mL/min, and the wavelength was 230 nm. The mobile
phase (50mM KH2PO4 buffer at pH 2.1:acetonitrile) for a-methylcinnamic
acid was 55:45 v/v, that for 2,4-dihydroxybenzoic acid, 3,4-dihydroxybenzoic
acid, 4-hydroxy-3-methoxybenzoic acid, 3,4-dihydoroxycinnamic acid and
4-hydroxyphenoxyacetic acid was 90:10 v/v, and that for the other benzoic,
phenoxyacetic and cinnamic acid derivatives was 70:30 v/v. The calibration
curves of the benzoic and cinnamic acid derivatives were linear over the
concentration range of 0.1-20 nmol/mL (r=0.999) and coefficient of variations
were lower than 4%.
Protein concentration was determined using a Bio-rad dye reagent
(Richmond, CA) with bovine serum albumin as the standard.
Statistical analyses
The data were analyzed by Scheffe’s multiple comparison test after the
analysis of variance using the Statcel 2 program, and differences with p values
< 0.05 were considered to be significant. Data are shown as mean ± standard
error (S.E.).
RESULTS
Comparison of the
uptake of benzoic acid derivatives and their inhibitory effects on the uptake
of benzoic acid
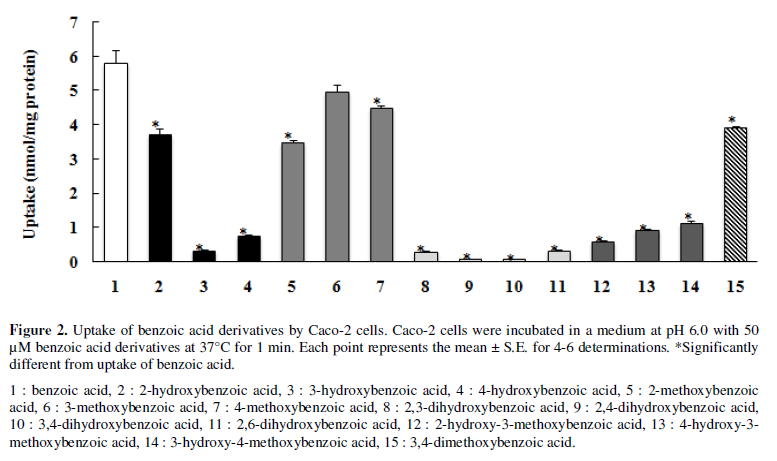
Caco-2 cells were incubated with 50 µM benzoic acid derivatives (Figure 1) at pH 6.0 for 1 min, and the uptake of the benzoic acid derivatives was compared with that of benzoic acid (parent compound) (Figure 2). Although mono-hydroxylation of the benzene ring at the ortho (o)-, meta (m)- and para (p)-position (chemical structures are shown in Figures 1, 2 and 3 respectively) significantly decreased uptake, the decreases induced by m- and p-hydroxylation were more profound than that induced by o-hydroxylation (The uptake of m-, p- and o-hydroxybenzoic acids was 0.3, 0.8 and 3.7 nmol/mg protein, respectively). The uptake of mono-methoxy benzoic acids at the m- and p-position (6,7) (5.0 and 4.5 nmol/mg protein) was marginally lower than that of the parent compound (5.8 nmol/mg protein), but that at o-substitution (5) was significantly lower (3.5 nmol/mg protein). Di-hydroxylation (8-11) resulted in marked decrease, with the differences in uptake due to substitution position being negligible (0.1-0.3 nmol/mg protein). The methoxylation of one of the hydroxyl groups (12-14) increased uptake.
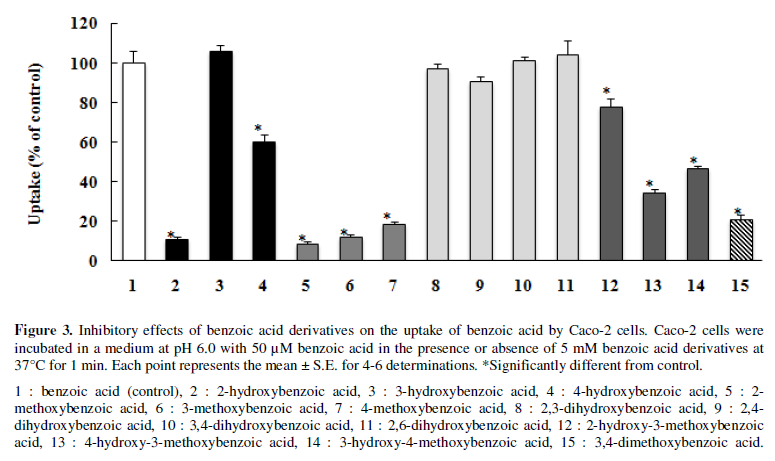
Caco-2 cells were coincubated with 50 µM benzoic acid and 5 mM benzoic
acid derivatives at pH 6.0 for 1 min (Figure
3). Coincubation with mono-hydroxyl benzoic acid at the o-position (2) profoundly decreased the
uptake of benzoic acid (89%) and that at the p-position (4) significantly decreased the uptake of benzoic acid
(40%), while that at the m-position
(3) did not affect uptake at all. Coincubation with di-hydroxyl benzoic acid at
different positions (8-11) did not decrease the uptake of benzoic acid.
Coincubation with benzoic acid derivatives containing mono-methoxy and
mono-hydroxyl groups (12-14) significantly decreased the uptake of benzoic acid
(21 to 65%), and that with derivatives containing di-methoxy groups (15)
further decreased uptake (75%).
Comparison of the
uptake of cinnamic acid and phenoxyacetic acid derivatives and their inhibitory
effects on the uptake of benzoic acid
Caco-2 cells were incubated with 50 µM cinnamic acid derivatives (Figure 4) at pH 6.0 for 1 min, and
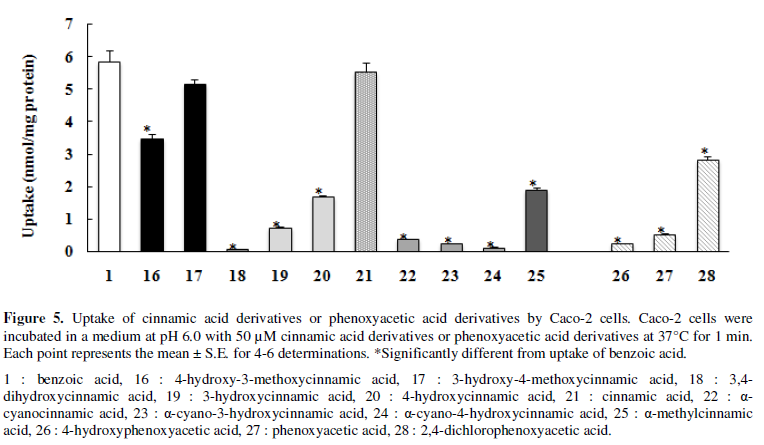
their uptake was compared with the uptake of cinnamic acid (21) (Figure 5). The uptake of cinnamic acid
derivatives containing cyano group, α-cyanocinnamic acid (22),
α-cyano-3-hydroxycinnamic acid (23) and α-cyano-4-hydroxycinnamic acid (24),
was very low (0.1-0.4 nmol/mg protein). The uptake of α-methylcinnamic acid
(25) (1.9 nmol/mg protein) acid was significantly lower than that of cinnamic
acid (21) (5.5 nmol/mg protein), but significantly higher than that of
α-cyanocinnamic acid (22) and its derivatives (23, 24).
The mono-hydroxylation of the benzene ring of cinnamic acid at the m- or p-position (19,20) resulted in significant decreased in the uptake
(0.7 and 1.7 nmol/mg protein, respectively). Di-hydroxylation at the m- and p-positions (18) resulted in further decreases, whereas the
methoxylation of one of the hydroxy groups at the m- or p-position (16, 17)
increased uptake (the uptake of those were 0.1, 3.5 and 5.2 nmol/mg protein,
respectively).
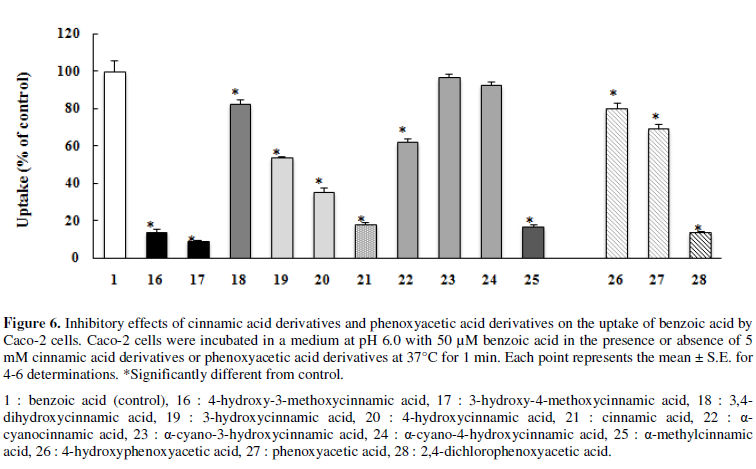
Caco-2 cells were coincubated with 50 µM benzoic acid and 5 mM cinnamic
acid derivatives at pH 6.0 for 1 min (Figure
6). Coincubation with α-cyano-3-hydroxycinnamic acid (23) and α-cyano-4-hydroxycinnamic
acid (24) did not decrease the uptake of benzoic acid, while coincubation with
α-cyanocinnamic acid (22) significantly decreased the uptake of benzoic acid by
38% and that with α-methylcinnamic acid (25) profoundly decreased the uptake by
83%.
The mono-hydroxylation of the benzene ring at the m- and p-positions
significantly decreased the uptake by 46% (19) and 64% (20), respectively.
Di-hydroxylation at the m- and p-positions marginally decreased uptake
by 17% (18), and the methoxylation of the hydroxy group at the m- or p-position (16,17) profoundly decreased the uptake of benzoic acid
by 86 and 90%, respectively.
The uptake of phenoxyacetic acid
(27) was low and that of 4-hydroxyphenoxyacetic acid (26) was even lower (Figure 5). Coincubation with
phenoxyacetic acid (27) or 2,4-dichlorophenoxyacetic acid (2,4-D) (28)
significantly decreased the uptake of benzoic acid (31 and 86%, respectively), whereas that with 4-hydroxyphenoxyacetic
acid (26) marginally decreased uptake (20%) (Figure 6).
Competitive
inhibition of cinnamic and 4-hydroxycinnamic acid on benzoic acid uptake
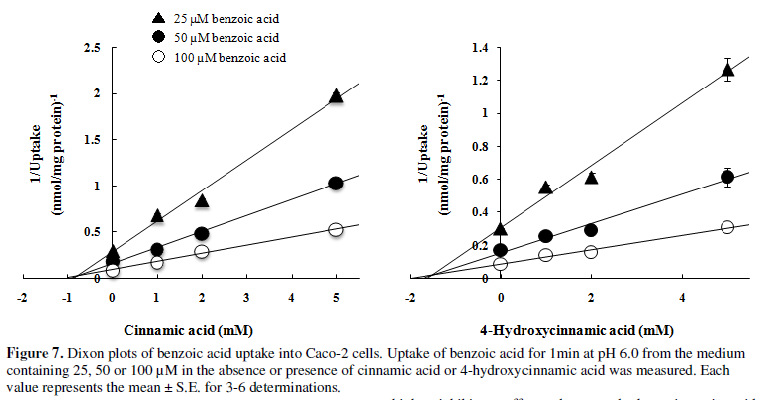
Inhibitory effects of cinnamic and 4-hydroxycinnamic acids (20,21) on benzoic
acid uptake by Caco-2 cells were analyzed using Dixon plots (Figure 7). Caco-2 cells were incubated
in a medium at pH 6.0 for 1min at 37°C with 25, 50 or 100 µM benzoic acid in
the absence or presence of cinnamic acid or 4-hydroxycinnamic acid (1, 2 or 5
mM). Both cinnamic acid and 4-hydroxycinnamic acid inhibited the uptake of
benzoic acid competitively, with apparent Ki
values of 0.8 and 1.5 mM, respectively.
DISCUSSION
The pKa of benzoic acid, and o-,
m- and p-hydroxybenzoic acids (2-4) are 4.2, 2.98, 4.08 and 4.57,
respectively [20], and the formation of internal hydrogen bonds between the
1-carboxyl and 2-hydroxyl groups contributing to the low pKa of the o-hydroxybenzoic acid [21]. The uptake
of o-hydroxybenzoic acid (Figure 2) and its inhibitory effect on
benzoic acid uptake (Figure 3) were
the highest among the o-, m- and p-hydroxybenzoic acids. The formation of internal hydrogen bonds
may be a possible reason for the highest uptake and inhibitory effect of o-hydroxybenzoic acid. On the other
hand, the uptake and inhibitory effect of m-hydroxybenzoic
acid were the lowest among the three isomers (Figures 2 and 3), which was at variance with the order of the pKa
values. A similar order was observed for the inhibitory effects of
mono-hydroxybenzoic acids (m- < p-< o-) on the uptake of MCPA (a phenoxyacetic acid derivative) [15]
and the inhibitory effects of m- and p-hydroxybenzoic acids on the transport
of salicylic acid [22]. In contrast, Konishi et al. [7] examined the inhibitory
effects of o-, m- and p-hydroxycinnamic
acids on fluorescein transport, and reported that m-hydroxycinnamic acid had a higher inhibitory effect whereas o-hydroxycinnamic acid had no inhibitory
effect (o- < p- < m-). The reason
for this contradiction remains unclear; however, it is speculated that
hydroxylation of the benzene ring may change MCT(s) affinity.
The uptake of di-hydroxybenzoic acids (8-11) and di-hydroxycinnamic
acid (18) were markedly lower than that of mono-hydroxybenzoic acids (2-4) and
mono-hydroxycinnamic acids (19,20), respectively, and the inhibitory effects of
di-hydroxybenzoic acids (8-11) and di-hydroxycinnamic acid (18) on benzoic acid
uptake were markedly lower than those of the respective mono-hydroxy compounds.
Hydroxylation, particularly di-hydroxylation, decreased the affinity for MCTs,
probably due to decreased lipophilicity. In agreement with our results, Konishi
et al. [7] reported di-hydroxylated benzoic acids (8,10,11) and
3,4-dihydroxycinnamic acid (18) did not have an inhibitory effect on
fluorescein transport (MCT substrate), while the respective mono-hydroxylated
compounds did inhibit fluorescein transport. On the other hand, that
mono-chloro- and di-chlorosubstitution of benzoic acids did not affect uptake,
whereas coincubation with those chlorobenzoic acids, except for
2,6-dichlorobenzoic acid, markedly decreased the uptake of benzoic acid
independent of the chloro-substitution position [18]. Among the
dihydroxybenzoic acids (8-11), we expected 2,6-dihydroxybenzoic acid (11) to
show the lowest level of uptake based on our previous report of the steric
hindrance associated with the 2,6-dichlorosubstitution of the benzoic ring limiting
MCT access to the carboxylic group [18]. However, the uptake of
dihydroxybenzoic acids investigated was too low to allow us to identify the steric hindrance of 2,6-dihydroxybenzoic
acid.
The uptake of 2-hydroxy-3-methoxybenzoic acid (12), 4-hydroxy-3-methoxybenzoic
acid (13) and 3-hydroxy-4-methoxybenzoic acid (14) was slightly but
significantly higher than that of 2,3-, 2,4, and 3,4-dihydroxybenzoic acid
(8-10), respectively (Figure 2), and
the inhibitory effects of those methoxybenzoic acids (12-14) on benzoic acid
uptake were significantly higher (Figure
3). A similar tendency due to the presence of a methoxy group was also
found in the cinnamic acid derivatives: The uptake of
3-hydroxy-4-methoxycinnamic acid (17) and 4-hydroxy-3-methoxycinnamic acid (16)
was higher than that of 3,4-dihydroxycinnamic acid (18) (Figure 5), and the inhibitory effect of these methoxycinnamic acids
(16,17) was higher than that of 3,4-dihydroxycinnamic acid (18) (Figure 6). The substitution of the
hydroxy group with a methoxy group could increase the uptake via MCTs.
The uptake of 3,4-dihydroxycinnamic acid (18) was markedly lower than
that of 3- and 4-monohydroxycinnnamic acid (19,20) as well as
4-hydroxy-3-methoxycinnamic acid (16) and 3-hydroxy-4-methoxycinnamic acid
(17), and the inhibitory effect of 3,4-dihydroxcinnamic acid (18) was
significantly lower than that of the other acids (16,17,19,20). Saito et al.
[9] compared the inhibitory effects of 4-hydroxycinnnamic acid (20),
3,4-dihydroxycinnamic acid (18) and 4-hydroxy-3-methoxycinnamic acid (16) on
the uptake of nateglinide (a possible substrate for MCTs or MCT-like
transporters) by Caco-2 cells, and found that the non-competitive inhibition of
3,4-dihydroxycinnamic acid (18) with a higher Ki value, in comparison with the competitive inhibition
of 4-hydroxycinnnamic acid (20) and 4-hydroxy-3-methoxycinnamic acid (16). The
internal hydrogen bonds between the 3- and 4-hydroxyl groups on the benzene
ring may be one reason for these phenomena [23]. Further study of
2,3-,2,4-,2,5- and 3,5-dihydroxylcinnamic acids is necessary to confirm the
effect of internal hydrogen bounds.
The uptake of 4-hydroxyphenoxyacetic acid (26) was lower than that of
phenoxyacetic acid (27: PA) and 2,4-dichlorophenoxyacetic acid (28: 2,4-D) (Figure 5), and the inhibitory effect of
4-hydroxyphenoxyacetic acid on benzoic acid uptake was also lower (Figure 6). Like benzoic acid and
cinnamic acid, the hydroxylation of benzene ring could decrease the MCT(s)
affinity. The pKa of 4-hydroxyphenoxyacetic acid was 4.5 and that of
phenoxyacetic acid and 2,4-D were 4.28 and 2.64, respectively. According to our
previous report [16], the uptake of phenoxyacetic acids increased in the order
of chloride substituent and lipophilicity (PA < 4-CPA < 2,4-D = MCPA <
2,4,5-T), with the pKa values of those compounds being 4.28, 4.19, 2.64, 3.07
and 2.88, respectively. The uptake of 4-hydroxyphenoxyacetic acid and its pKa
are in good agreement with this order. These results strengthen our hypothesis
that the uptake of phenoxyacetic acid derivatives is mediated by the same MCTs
as benzoic acid, at least in part, and the affinity of those derivatives for
MCTs is determined by their lipophilicity and pKa values [16].
The uptake of α-cyanocinnamic acid derivatives was trace (22-24) (Figure 5), and these derivatives
decreased slightly or not at all the uptake of benzoic acid (Figure 6). In contrast, the uptake of
α-methylcinnamic acid (25) was significantly higher than that of the
α-cyanocinnamic acid derivatives (22-24) (Figure
5), and this compound significantly decreased the uptake of benzoic acid (Figure 6). The α-cyano group of
cinnamic acids may inhibit the accessibility of the carboxyl group to the MCTs.
The uptake of α-cyano-4-hydroxycinnamic acid (24) and
α-cyano-3-hydroxycinnamic acid (23) was lower than that of α-cyanocinnamic acid
(22), although the uptake of all of those compounds was low (Figure 5). The inhibitory effects of
α-cyano-4-hydroxycinnamic acid (24) and α-cyano-3-hydroxycinnamic acid (23) on
the uptake of benzoic acid were slight, whereas that α-cyanocinnamic acid (22)
was significant (Figure 6).
Hydroxylation of cinnamic acids at the benzene ring appears to slightly weaken
their affinity for the MCTs that mediate benzoic acid uptake. In agreement, the
Dixon plot (Figure 7) suggests that
4-hydroxycinnamic acid (24) has a higher Ki
value than cinnamic acid (21) in terms of benzoic acid uptake.
Available information on MCT subtypes is somewhat controversial. MCT1-4
are characterized as proton-linked MCTs among 14 MCT subtypes [1], and Caco-2
cells expressed MCT1, MCT3, MCT4 and MCT6 with MCT1 being the most abundant
[11]. α-Cyano-cinnamic acid analogs are the inhibitors of MCT1, MCT2 and MCT4,
and the inhibitory effect of α-cyano-4-hydroxycinnamic acid (CHC) on MCT1
activity is about ten times higher than that of other α-cyano-cinnamic acids
[1]. However, the uptake of benzoic acid and phenoxyacetic acid derivatives
could be mediated via proton-linked MCTs and coincubation with CHC hardly
decreased their uptake. Kuwayama et al. [24] reported the similar results that
fluorescein uptake by Caco-2 cells could be mediated via proton-lined MCTs
which were distinct from MCT1-4. Further study is necessary to clarify the
contradicted information in the MCT subtypes and elucidated the MCT subtype
mediated the benzoic acid, phenoxylic acid derivatives and fluorescein.
In conclusion, the hydroxylation of benzoic acid, cinnamic acid and
phenoxyacetic acid decreased their uptake via Caco-2 cells, and the uptake of
those compounds was mediated via common MCTs at least in part. Further, the
α-cyano group of cinnamic acid decreased the affinity of their carboxyl groups
to MCT(s).
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
This study was supported by Grants-in-Aid from Research for Promoting
Technological Seeds (T.E.: H20, 01-040) and from Japan Society for the
Promotion Science (O.K.: C24614012, T.E.: C16K00863).
1. Halestrap
AP (2013) Monocarboxylic acid transport. Compr Physiol 3: 1611-1643.
2. Halestrap
AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs)
to aromatic amino acid transporters and beyond. Pflugers Arch 447: 619-628.
3. Tsuji A, Takanaga H, Tamai I, Terasaki T (1994) Transcellular
transport of benzoic acid across Caco-2 cells by a pH-dependent and
carrier-mediated transport mechanism. Pharm Res 11: 30-37.
4. Li
YH, Ito K, Tsuda Y, Kohda R, Yamada H, et al. (1999) Mechanism of intestinal absorption
of an orally active β-lactam prodrug: uptake and transportof carindacillin in
Caco-2 cells. J Pharmacol Exp Ther 290: 958-964.
5.
Okamura A, Emoto A, Koyabu N, Ohtani H, Sawada Y (2002) Transport and
uptake of nateglinide in Caco-2 cells and its inhibitory effect on human
monocarboxylate transporter MCT1. Br J Pharmacol 137: 391-399.
6.
Konishi Y, Shimizu M (2003)
Transepithelial transport of ferulic acid by monocarboxylic acid transporter in
Caco-2 cell monolayers. Biosci Biotechnol Biochem 67: 856-862.
7. Konishi Y, Kubo K, Shimizu M (2003) Structural
effects of phenolic acids on the transepithelial transport of fluorescein in
Caco-2 cell monolayers. Biosci Biotechnol
Biochem 67: 2014-2017.
8.
Choi JS, Jin MJ, Han HK (2005) Role of monocarboxylic acid transporters
in the cellular uptake of NSAIDs. J Pharm Pharmacol 57: 1185-1189.
9. Saito
Y, Itagaki S, Otsuka Y, Kobayashi Y, Okumura H, et al. (2005) Substrate
specificity of the nateglinide/H+ cotransport system for phenolic
acids. J Agric Food Chem 53: 6100-6104.
10.
Hilgers AR, Conradi RA,
Burton PS (1990) Caco-2 cell monolayers as a model for drug transport across
the intestinal mucosa. Pharm Res 7: 902-910.
11. Hadjiagapiou
C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K (2000) Mechanism(s) of butyrate
transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol
Gastrointest Liver Physiol 279: G775-780.
12. Halestrap
AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family:
structure, function and regulation. Biochem J 343: 281-299.
13. Konishi
Y, Hagiwara K, Shimizu M (2002) Transepithelial transport of fluorescein in
Caco-2 cellmonolayers and use of such transport in in vitro evaluation of
phenolic acid availability. Biosci Biotechnol Biochem 66: 2449-2457.
14. Itagaki
S, Kobayashi Y, Otsuka Y, Kubo S, Kobayashi M, et al. (2005) Food-drug
interaction between ferulic acid and nateglinide involving the fluorescein/H+
cotransport system. J Agric Food Chem 53: 2499-2502.
15. Kimura
O, Tsukagoshi K, Endo T (2008) Uptake of 4-chloro-2-methylphenoxyacetic acid
(MCPA) from the apical membrane of Caco-2 cells by the monocarboxylic acid
transporter. Toxicol Appl Pharmacol 227: 325-330.
16. Kimura
O, Tsukagoshi K, Endo T (2009) Uptake of phenoxyacetic acid derivatives into
Caco-2 cells by the monocarboxylic acid transporters. Toxicol Lett 189:
102-109.
17. Kimura
O, Tsukagoshi K, Hayasaka M, Endo T (2012) Uptake of triclopyr
(3,5,6-trichloro-2-pyridinyloxyacetic acid) and dicamba
(3,6-dichloro-2-methoxybenzoicacid) from the apical membranes of the human
intestinal Caco-2 cells. Arch Toxicol 86: 55-61.
18. Tsukagoshi K, Kimura O, Endo T (2014) Steric
hindrance of 2,6-disubstituted benzoic acid derivatives on the uptake via
monocarboxylic acid transporters from the apical membranes of Caco-2 cells. Pestic
Biochem Physiol 111: 38-42.
19. Kimura
O, Haraguchi K, Ohta C, Koga N, Kato Y, et al. (2014) Uptake of aristolochic
acid I into Caco-2 cells by monocarboxylic acid transporters. Biol Pharm Bull 37:
1475-1479.
20. Jencks
WP, Regenstein J (2010) Ionization constant of acids and bases. Handbook of
Biochemistry and Molecular Biology. CEC Press, Boca Raton.
21.
Dunn GE, Penner TL
(1966) Effect of intramolecular hydrogen bonding on the relative acidities of
substituted salicylic acids in benzene solution. Can J Chem 45: 1699-1706.
22. Takanaga H, Tamai I, Tsuji A (1994) pH-Dependent and
carrier-mediated transport of salicylic acid across Caco-2 cells. J Pharm
Pharmacol 46:567-570.
23. Ishimitsu
T, Hirose S (1977) Microscopic and dissociation constants of
3,4-dihydroxyphenylpropoinic acid and related compounds, and 3,4-dihydroxyphenylalanine
(DOAP). Talanta 24: 555-560.
24. Kuwayama
K, Miyauchi S, Tateoka R, Abe H, Kamo N (2002) Fluorescein uptake by a
monocarboxylic acid transporter in human intestinal Caco-2 cells. Biochem
Pharmacol 63: 81-88.