829
Views & Citations10
Likes & Shares
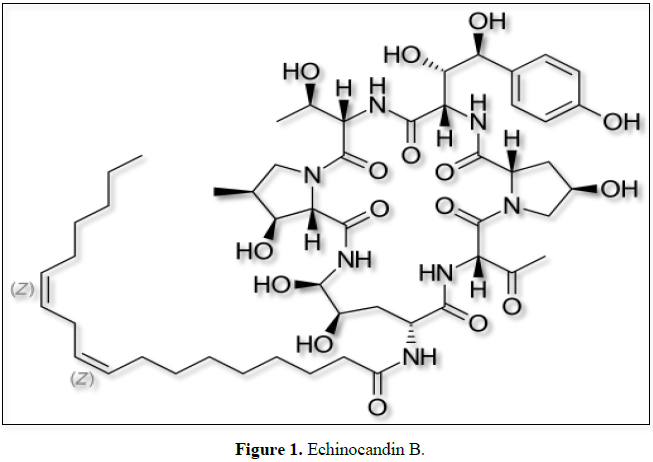
Echinocandin B nucleus an intermediate used in the
production of Anidulafungin a new class of antifungal agent used to treat
chronic fungal infections. Echinocandin B gets converted to Echinocandin B
Nucleus through an enzymatic bioconversion called deacylation by the enzyme
Acylase also called as deacylase. A rapid bioconversion screening method was
performed to select Actinomycete capable of producing Echinocandin B acylase
amongst the group of Actinomycetes strains screened. A total of 140 strains of
Actinomycete isolates were used for the preliminary qualitative plate assay
with 53 strains selected positive for acylase production followed by two
strains selected for quantitative assay. The selected strains were used for
optimizing the bioconversion by varying parameters such as pH, temperature,
feeding time temperature and substrate concentration in lab scale (50 ml
medium) shake flask studies. The effect of temperature on conversion rate was
also optimised. The optimised conditions were also tested in higher volume (500
mL medium) shake flasks which showed a 3 fold increase in conversion rate when
compared to that of lab scale shake flask trials with Actinoplanes utahensis as the control.
Keywords: Antifungals, Echinocandin
B, Anidulafungin, Acylase, Aspergillus
nidulans
INTRODUCTION
The
population of cancer patients, transplant recipients and other individuals
receiving immunosuppressive treatment are at greater risk for fungal infections
because of their weak immune system and the chronic nature of diseases.
Therefore, treatment of such invasive fungal infections has created a major
challenge for health care professionals. Antibiotics are known to target
important cellular functions or growth processes of microbes. Echinocandins are
synthetically modified non ribosomal cyclic hexapeptides conjugated with a
fatty acid (Figure 1). They have a
unique mode of action against pathogenic fungi by non-competitively inhibiting
the β-1, 3 D-glucan synthase, a key enzyme involved in the synthesis of β-1, 3
D-glucan, an essential structural component of fungal cell wall [1-4]. They show fungistatic activity
against Aspergillus spp. and
fungicidal activity against several Candida
spp. including strains that are fluconazole resistant. Caspofungin, Micafungin
and Anidulafungin are the three important semisynthetic Echinochandins [5-7].
All have low oral bioavailability and therefore distribute well into tissues
except in CNS and eye. Anidulafungin is a new echinocandin antifungal agent
used extensively for the treatment of oesophageal candidiasis and candidemia.
It is unique in that it undergoes chemical degradation in bile rather than via
hepatic metabolism with longer half-life than Caspofungin or Micafungin.
Anidulafungin is named after the fungus, Aspergillus
nidulans, the first organism reported to produce Echinocandin B (ECB) [8].
Enzymatic deacylation of ECB to a cyclic hexapeptide without a linoleoyl side
chain (Echinocandin B nucleus) and by subsequent chemical reacylation leads to
the formation of anidulafungin [9-12].
Acyl peptides are generally unstable to chemical deacylation and therefore
enzymatic deacylation is widely accepted. Deacylation of Echinochandin B to
Echinochandin B nucleus is catalysed by the acylase (also named as deacylase).
This enzyme is currently identified from Actinoplanes utahensis NRRL
12052. It is a membrane-associated heterodimer composed of 63 kDa and
The present
study was initiated with an aim to identify an organism having a lipopeptide,
acylase, with high specificity towards Echinocandin B. Therefore, a rapid
screening protocol was undertaken with bacteria belonging to Actinomycetes,
isolated from tropical soil. The selected species was evaluated for its
efficiency to bio convert ECB to ECB nucleus and the cultural and environmental
parameters for the bioconversion was optimized.
MATERIALS AND METHODS
Chemicals and reagents
All the
chemicals and reagents used were of analytical grade and were purchased from
Merck. Echinocandin B was prepared in-house by the fermentation of Aspergillus nidulans [13].
Microorganisms and culture conditions
All the
cultures used for screening were isolated from natural sources (Soil, water or
endophytes) across Karnataka, India. The cultures thus isolated were stored as
glycerol stocks at the Biocon India Culture Collection (BICC). Totally, 140
Actinomycetes were cultured on ISP-4 (from Difco medium) plates at 28°C for one
week. The control organism Actinoplanes
utahensis (NRRL 12052) a type strain obtained from Agricultural research
service culture collection (NRRL), Peoria, Illinois.
Enzyme screening studies
Qualitative plate assay for screening of Acylase
producing strains: In the first round of screening, all the 140
Actinomycetes were inoculated onto fresh ISP-4 plates containing 1 mg/ml of
Echinocandin B. The plates were incubated at 30°C for 7 days and at the end of
the incubation, the plates were overlaid with 5 mL of soft agar containing 103
CFU/mL of Candida albicans.
[14]. The overlaid plates were further incubated at 30°C for 24 h and the
plates with the growth of C. albicans
were considered as positive for acylase.
Quantitative assay for
production of acylase under submerged fermentation (SMF): Fifty-three strains positive for acylase were selected from
quantitative plate assay. These strains were further inoculated into
Streptomyces seed media (4% glucose, 1% yeast extract, 0.1% CaCO3,
pH adjusted to 6.5 and 25 mL of the media was dispensed into 250 mL flask) and
incubated at 28°C for 3 days in an orbital shaker at 230 rpm. Three mL of the
inoculum was transferred into production medium (4% glucose, 1% Soya peptone,
1% yeast extract, 1% KH2PO4, 0.5% K2HPO4,
0.1% KCL, 0.01% calcium carbonate, pH adjusted to 7.0 and 30 mL of the media
was dispensed into 250 mL flask) containing filter sterilized Echinocandin B
(dissolved in methanol) at a concentration of 3.0 g/L and incubated at 30°C for
3 days in an orbital shaker at 230 rpm. Samples were withdrawn at an interval
of 24 h from the time of inoculation, centrifuged at 3000 rpm for 15 min and
the supernatant was analysed for the formation of Echinocandin B nucleus by
HPLC. Agilent Poroshell EC18 (150*4.6 mm, 2.7 µm pore size) column with KH2PO4
buffer and HPLC grade Acetonitrile mobile phases used for HPLC analysis using
Echinocandin B and Echinocandin B nucleus from standard commercial sources
[15].
The
production of Acylase in Streptomyces seed and production media (devoid of the
substrate – Echinocandin B) were analysed by separating the cells by
centrifugation at 3000 rpm for 15 min. 1 g/L of Echinocandin B (in 50 mM
phosphate buffer at pH 7) was added to the cell free supernatant and incubated
for 24 h at 30°C. The reaction mixture was centrifuged at 3000 rpm for 15 min
and the supernatant was analysed in HPLC (as described above) to detect
Echinocandin B nucleus.
Optimisation studies for bioconversion of Echinocandin B
to Echinocandin B nucleus
For the
optimisation studies different parameters which affect the bioconversion of
Echinocandin B to Echinocandin B nucleus were studied which include pH,
temperature, substrate concentration, feeding time and media volume. Two
strains positive for acylase production coded as BICC-8848, BICC-8547 with a
control organism Actinoplanes utahensis
in the production media were studied under submerged fermentation conditions.
These strains were inoculated into Streptomyces seed media and incubated at
28°C for 3 days in an orbital shaker at 230 rpm. Three mL of the
inoculum was transferred into production medium and incubated at 30°C for 3
days in an orbital shaker at 230 rpm. Samples (1 mL) were withdrawn at an
interval of 24 h from the time of inoculation till end of incubation,
centrifuged at 3000 rpm for 15 min and the supernatant was analysed for the
formation of Echinocandin B nucleus by HPLC (as described in section 2.2.2)
[15].
Effect of pH
The
production medium was adjusted to pH ranges (5.5, 6.0, 6.5, 7.0 and 7.5) with
10% H3PO4/10% NaOH before sterilization. The sterile
medium was supplemented with the substrate Echinocandin B (3 g/L) before they
were inoculated with the cultures.
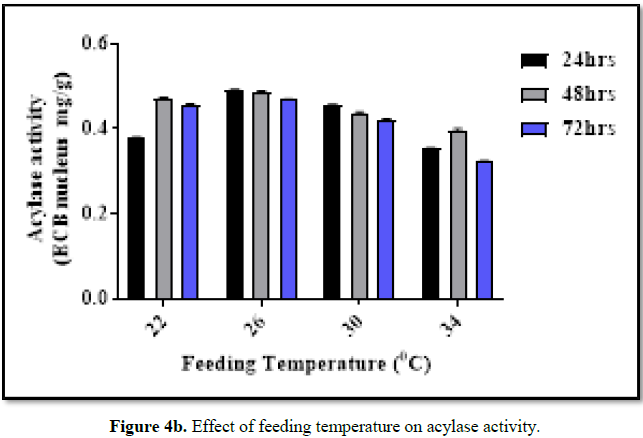
Effect of temperature
The
inoculated production medium was incubated at different temperature (22°C, 26°C,
30°C and 34°C) for 72 h in an orbital shaker at 230 rpm. Samples were withdrawn
at regular interval of time (every 24 h) and analysed by HPLC.
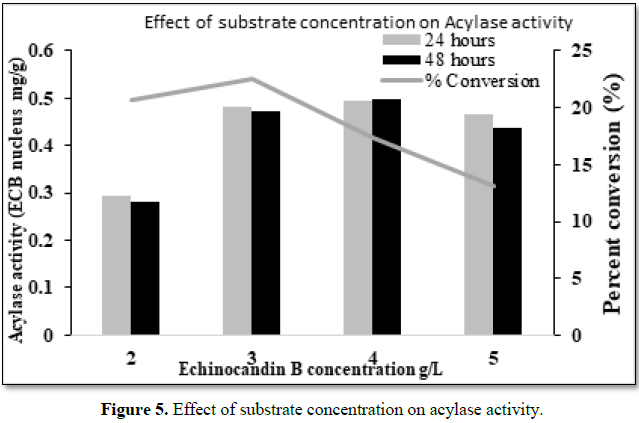
Effect of substrate concentration
The
inoculated production medium was fed with Echinocandin B at different
concentrations (2, 3, 4, 5 g/L) incubated at 26°C for 72 h in an orbital shaker
at 230 rpm. Samples were withdrawn at regular interval of time (every 24 h) and
analysed by HPLC.
Effect of feeding time of substrate
The
inoculated production medium was incubated at 26°C in an orbital shaker at 230
rpm and fed with Echinocandin B (3 g/L) at regular intervals from the time of
inoculation (0, 24, 48, 72, 96, 120 h). Samples were withdrawn at regular
interval of time (every 24 h) and analysed by HPLC.
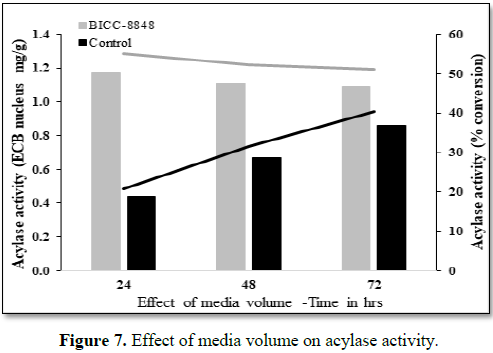
Effect of media volume on bioconversion
In general,
the acylase activity varies depending on the volume of the medium used in the
shake flasks. The pre-grown culture in the seed medium was inoculated into
production media (500 mL in 2000 mL conical flask adjusted to pH 7.0) and
incubated at 30
Molecular level Identification of the
selected Actinomycete
Two strains
(BICC-8848 and BICC-8547) were identified to produce the enzyme. Genomic DNA
from the selected Actinomycetes was extracted; 16S rRNA gene was amplified
using 16 S universal primers. The amplified PCR product was sequenced, the
obtained sequence was checked in NCBI BLAST analyser and identification is made
based on the maximum homology to the available sequence in the NCBI database
[16].
RESULTS
Qualitative plate assay for screening for Echinocandin B
acylase producing strains
A total of
140 isolates belonging to Actinomycetes were screened by plate assay method and
53 isolates (37%) were found to be positive for converting the Echinocandin B,
an antifungal compound, into a non-effective molecule, Echinocandin B Nucleus.
Growth of Candida albicans around the
Actinomycete colony in the soft agar was an indication of bioconversion.
Satoshi et al. [14] screened 3300 Actinomycetes and 500 fungal strains and were
able to select 22 Actinomycetes (0.6% only) and 2 fungal strains (0.06% only)
positive for the bioconversion.
Quantitative assay for production of acylase under
submerged fermentation (SMF)
Actinomycetes
isolates (53 in number) selected from the plate assay method of screening were
subjected to second round of screening by submerged fermentation, for the
selection of strains with higher Acylase activity. Two Streptomyces species
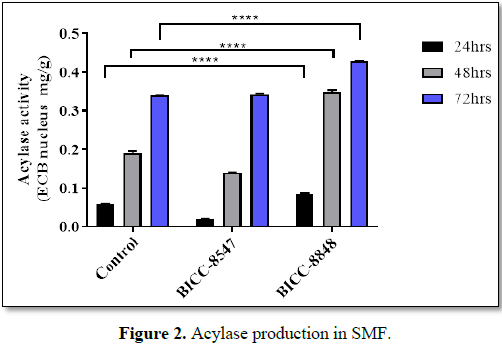
were selected, coded as BICC 8547 and BICC 8848. Among the two, BICC 8547
showed acylase activity equivalent to that of the control micro-organism (Figure 2). However, BICC 8848 showed
higher activity (25 to 30% more) of Acylase compared to the control organism
and therefore selected for further studies. The cell free supernatant fed with
1 g/L Echinocandin B showed very poor conversion.
The acylase
activity is directly proportional to the conversion of ECB to ECB nucleus. Therefore,
the activity of the acylase was determined by the titre value of ECB nucleus
which was quantified by HPLC analysis
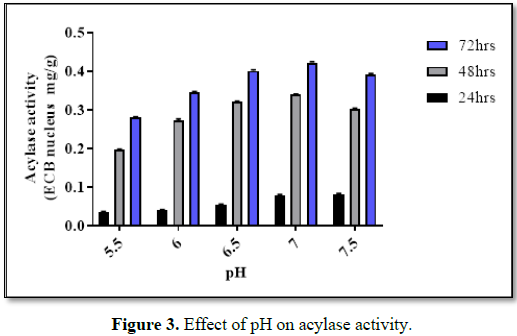
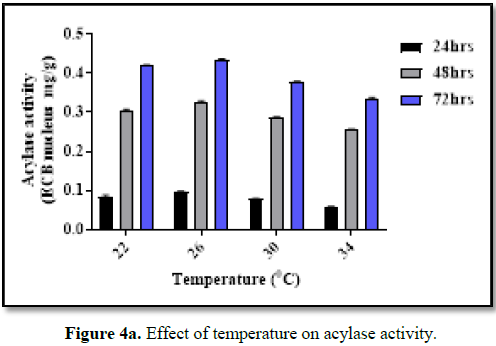
Effect of pH and temperature
The optimal
pH and temperature for acylase activity (for bioconversion) of BICC-8848 was
found to be optimal at pH of 7.0 and at temperature of 26
Effect of substrate concentration
Any
substrate beyond a particular concentration can be toxic to cells or can reduce
the activity of the enzyme. Echinocandin B was fed at different concentrations
to production broth and we could observe higher conversion rate at 4 g/L, but,
however, the conversion rate was decreased when the concentration of the
substrate was increased (Figure 5).
Effect of feeding time of substrate on conversion rate
In the
process of determining the optimal pH and temperature for bioconversion, it was
observed that the impurity level was higher than the control. Therefore, an
attempt was made to decrease the level of impurity by adding the substrate at
different duration of growth of the culture in production media. Interestingly,
it was observed that BICC-8848 was able to convert the substrate in 24 h by
lowering the impurity. However, it was also observed that the concentration of
the product decreased from 48 h onwards with increased impurity (Figure 6).
Effect of media volume on bioconversion
An attempt
was made to evaluate the efficiency of the conversion with respect to volume of
the media taken. The percentage conversion was observed to be generally lower
in lower volume of broth, therefore trial was performed in higher volume, i.e.,
500 mL of media (in 2 L conical flask) with the above set parameters and was
observed that the conversion rate was as high as 55.0% when compared to 22.5%
in 100 mL medium after 24 h of incubation. The conversion in BICC-8848 was
completed in 24 h while the conversion in control strain proceeded till 72 h
and the maximum conversion was 40.2% at the end of 72 h (Figure 7). It is evident that maximum conversion is achieved with
higher volume with respect to mass balance ratio.
Molecular level identification of culture
The
actinomycetes strain BICC-8848 when subjected to ribotyping match only 99% with
other Streptomyces species, showing only 98% homology to Streptomyces spp. B180, Streptomyces
parvus and Streptomyces sindenensis.
These Streptomyces spp. have not been
reported so far to possess the acylase activity and the strain Streptomyces sp. BICC-8848 is a strain
with better Acylase activity (better than Actinoplanes
utahensis NRRL 12052) which can be studied further with detailed taxonomic
studies such as genome sequencing and comparative studies to identify it till
species level.
GenBank
accession number SUB4743646 SEQ1 MK123323
DISCUSSION AND
CONCLUSION
Deacylation
is a critical step in the semi-synthetic production of various novel
lipopeptides. The enzymatic deacylation of Echinocandin B to Echinocandin B
nucleus is an important step followed by chemical reacylation with an optimized
acyl group to get a pharmacologically important antifungal drug, Anidulafungin
[17]. These steps are conveniently and efficiently carried out by microbes when
incubated with suitable substrate. The present investigation was taken up in
search of an Actinomycete with better acylase activity. The preliminary data
obtained in our present study revealed that the selected Actinomycete (Streptomyces spp. BICC-8848) possess acylase with good conversion rate (up to 55%
conversion rate compared to 22% in control organism) at standard growth
conditions than few Streptomyces and fungal organisms reported earlier. The molecular characteristics of our isolate of
Streptomyces show only 99% similarity to any of the species within the genus described
to date [18-20]. This species of Streptomyces could be a novel one, an
additional Actinomycete member, under the family of acylase producers.
Structural elucidation of acylase protein of this novel Streptomyces species
would be studied further, to understand its structure-function relationship [21].
ACKNOWLEDGMENT
The authors thank department of Microbiology and Biotechnology, Bangalore
University and Biocon Ltd.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
1. Wiederhold
NP, Lewis RE (2003) The echinocandin antifungals: An overview of the
pharmacology, spectrum and clinical efficacy. Expert Opin Investig Drugs 12:
1313-1333.
2. Denning
DW (2003) Echinocandin antifungal drugs. Lancet 362: 1142-1151.
3. Odds
FC, Brown AJ, Gow NA (2003) Antifungal agents: Mechanisms of action. Trends
Microbiol 11: 272-279.
4. Zaas
AK, Alexander BD (2005) Echinocandins: Role in antifungal therapy. Expert Opin
Pharmacother 6: 1657-1668.
5. Sucher
AJ, Chahine EB, Balcer HE (2009) Echinocandins: The newest class of
antifungals. Ann Pharmacother 43: 1647-1657.
6. Wagner
C, Graninger W, Presterl E, Joukhadar C (2006) The echinocandins: Comparison of
their pharmacokinetics, pharmacodynamics and clinical applications.
Pharmacology 78: 161-177.
7. Cleary
JD (2009) Echinocandins: Pharmacokinetic and therapeutic issues. Curr Med Res
Opin 25: 1741-1750.
8. Nyfeler
R, Keller-Schierlein W (1974) Metabolites of microorganisms 143 Echinocandin B,
a novel polypeptide-antibiotic from Aspergillus
nidulans var. echinulatus:
Isolation and structural components. Helv Chim Acta 57: 2459-2477.
9. Debono
M, Abbott BJ, Turner JR, Howard LC, Gordee RS, et al. (1988) Synthesis and
evaluation of LY121019, a member of a series of semisynthetic analogues of the
antifungal lipopeptide echinocandin Ba. Ann N Y Acad Sci 544: 152-167.
10. Onishi
J, Meinz M, Thompson J, Curotto J, Dreikorn S, et al. (2000) Discovery of novel
antifungal (1,3)-D-glucan synthase inhibitors. Antimicrob Agents Chemother 44:
368-377.
11. Petraitiene
R, Petraitis V, Groll AH, Candelario M, Sein T, et al. (1999) Antifungal
activity of LY303366, a novel echinocandin B, in experimental disseminated
candidiasis in rabbits. Antimicrob Agents Chemother 43: 2148-2155.
12. Uzun
O, Kocagöz S, Cetinkaya Y, Arikan S, Unal S (1997) In vitro activity of a new echinocandin, LY303366, compared with
those of amphotericin B and fluconazole against clinical yeast isolates.
Antimicrob Agents Chemother 41: 1156-1157.
13. Abbott
BJ, Fukuda DS; Eli Lilly and Company (1982) United States Patent 4322338.
14. Satoshi
U, Kazunori S, Nobutaka O, Masaru T, Michio Y, et al. (2010) Screening and
characterization of microorganisms with FR901379 acylase activity. J
Antibiotics 63: 65-70.
15. Ueda
S, Shibata T, Ito K, Oohata N, Yamashita M, et al. (2011) Cloning and
expression of the FR901379 acylase gene from Streptomyces sp. no. 6907. J Antibiotics 64: 169-175.
16. Rintala
H, Nvalainen A, Ronka E, Suutari M (2001) PCR primer targeting the 16s RNA gene
for the specific detection of Streptomyces. Mol Cell Probes 15: 337-347.
17. Barrett
D (2002) From natural products to clinically useful antifungals. Biochem
Biophys Acta 1587: 224-233.
18. Kauffman
CA, Carver PL (2008) Update on echinocandin antifungals. Semin Respir Crit Care
Med 29: 211-219.
19. Monk
BC, Goffeau A (2008) Outwitting multidrug resistance to antifungals Science
321: 367-369.
20. Paolo
B, Diana DG, Fabio F, Maurizio R (2007) Vanillin production using metabolically
engineered Escherichia coli under
non-growing conditions. Microbial Cell Factories 6: 13.
21. Wenwen
C, Erzheng S, Shujing Z, Yuhong R, Dongzhi W (2014) Characterization of a
nitrilase from Arthrobacter aurescens
CYC705 for synthesis of iminodiacetic acid. J Gen Appl Microbiol 60: 207-214.