535
Views & Citations10
Likes & Shares
Some drug companies recommend vitamin A supplements in
nutrition, as a means to raise immunity and decrease malaria prevalence. Data
of clinical trials collected in this field, however, are controversial. Plasmodium falciparum uses vitamin A for
its development. Helminths also use vitamin A and to some extent protect
against malaria. On the other hand, high concentrations in human serum and
liver are toxic. More disturbing is the fact that vitamin A and artemisinin are
highly antagonistic an antioxidant destroying an oxidant supposed to kill the
parasite.
Keywords: Vitamin A, Malaria,
Clinical trials, Human serum
INTRODUCTION
Vitamin A is
an essential nutrient for humans because it cannot be synthesized de novo. The molecule is involved in all
normal cellular proliferation and differentiation processes. Particularly,
vitamin A and some of its derivatives are required for several processes,
including embryogenesis, vision, reproduction, skeletal development and
maintenance of epithelial tissues. The vitamin is stored primarily in the
liver.
In this
document we will use indistinctly vitamin A for vitamin A, retinol or carotene.
Vitamin A clearly demonstrates hormetic effects: beneficial at low
concentrations, toxic at high doses with an optimal concentration which needs
to be defined from case to case.
Trials with
vitamin A supplements against malaria have given moderate or controversial
results. Randomized, placebo-controlled trials of prophylactic vitamin A
supplementation were run in northern Ghana. In the mortality study, 21,906
children were visited every 4 months over 2 years and in the morbidity study
1455 children were visited weekly for 1 year. There was no difference between
children supplemented with vitamin A and those given placebo in malaria
mortality rates or fever incidence based on reported symptoms. Malaria
parasitemia rates, parasite densities in children with a positive blood smear,
and rates of probable malaria illness also did not differ between treatment
groups. It is concluded that vitamin A supplementation had no impact on malaria
in this population [1-4].
A trial with
vitamin A supplementation in New-Guinea found reduced secretion of TNF,
upregulated CD36 expression and increased phagocytosis of Plasmodium falciparum parasitized erythrocytes.
Vitamin A
plays a role in immunity and protection against infectious diseases. Its role
reducing incidence of diarrhea and measles is known, but its role in relation
to malaria is unclear. A comprehensive, systematic literature search was
conducted on PubMed and Cochrane Library to identify randomized controlled
trials on the role of vitamin A during pregnancy and childhood for prevention
and treatment of malaria. Based on the meta-analysis, vitamin A supplementation
during pregnancy had no benefit for placental infection. Similarly, there was
no effect on peripheral parasitemia or episodes of new clinical malaria.
Preventive vitamin A supplementation in children younger than 5 years did not
reduce the incidence of peripheral parasitemia or malaria. Vitamin A as an
adjunct treatment for cerebral or severe malaria in children did not have
benefit on survival, fever resolution time, parasite clearance time or
incidence of neurological or other complications. The authors concluded that
vitamin A has no benefit for malarial infection either as prevention or
treatment in pregnancy or childhood based on RCT evidence [5].
Malarial infection is accompanied by
reductions in serum vitamin A concentrations from 120 mmol/l to 70 mmol/l (20
mg/dl). Reduced serum vitamin A levels are also found consistently in children
with malaria. Such observations have led to the suggestion that Plasmodium falciparum uses vitamin A from the host for
its metabolism; in fact, P. falciparum selectively absorbs vitamin A from host tissues. This selective uptake
of vitamin A accumulates in the parasites in a parasitemia-dependent manner and
increases with parasite maturation from the ring to the late trophozoite stage
[7].
The vitamin A
uptake of Plasmodium falciparum was investigated by culturing a standard
isolate of the parasite, at concentrations of the vitamin corresponding to
those normally present in human serum. Vitamin A accumulated in the parasites
in a parasitemia-dependent manner. And increased with parasite maturation from
the ring to the late-trophozoite stage, Vitamin A in the cytoplasm of late
trophozoites indicates that P.
falciparum may use vitamin A, from its human host, as an
antioxidant, to protect itself from oxidative stress [8].
Vitamin A
has a slight inhibitory effect on beta-hematin formation (Mutaz Ajkkawi,
personal communication).
Another critical issue is the interaction between vitamin A and
arginine. Arginine has antimalarial properties because it generates the strong
oxidant NO. Arginine therapy is beneficial in severe malaria in addition to
conventional antimalarial treatment. Low levels of arginine, the precursor for
nitric oxide, are common in patients with malaria. Research work has shown that
hypo-argenimia could be caused by vitamin A and that arginine or NO therapy
could be effective in malaria by inhibiting the actions of vitamin A [9].
Immunoglobulin E plays an important role in malaria prophylaxis.
Retinoic acid (RA, derived from vitamin A) markedly inhibited IgE starting at
concentrations of >10−14 mol/L for B cells and >10−10 mol/L for PBMC. Maximal inhibition of IgE production for B
cells was at 10−8 mol/L. Low
concentrations of RA inhibiting IgE synthesis (10−10 mol/L)
affected neither B-cell proliferation nor the production of IgA, IgG and IgM
[10].
Supplementation
with carotene plus Vitamin E effectively suppresses both the antigen-specific
and total IgE [11].
Apparent contradictions on the role of
vitamin A can eventually be resolved if we consider that vitamin A, while
essential in low concentration for numerous biological functions, is toxic at
higher concentrations; in addition, the merozoite-stage parasite spends several
days in the liver, the major storage organ for vitamin A, before invading the
erythrocytes. This suggests that serum vitamin concentrations are reduced in
malaria infection, in part from selective absorption of vitamin A by the
parasite and perhaps to a greater extent from impaired hepatic secretion of
vitamin A, since disturbed liver function is recognized in malaria [12-14].
It was also found that vitamin A accumulates
preferably in the Kuppfer cells of the liver, rather than in hepatic stellate
cells. Kuppfer cells are the entry port for sporozoites into the liver.
It may be hypothesized that the signs and symptoms of malaria are due to
the effects of vitamin A accumulated by the parasites in the host liver. It is
proposed that the parasites use the vitamin A, to invade the red blood cells;
the parasites egressing from the liver are packed with vitamin A. it is then
distributed via the transport of RBCs throughout the body in toxic
concentrations. Based on this hypothesis, hemolysis and anemia occur due to the
membranolytic actions of Vitamin A released from the parasites to invade the
RBCs. Other symptoms of the
disease, e.g. fever, headache, muscle aches, gastrointestinal symptoms,
seizures, coma, respiratory distress and retinopathy – may similarly reflect
parasite-induced vitamin A toxicity in the brain and other organs.
Toxicity of vitamin A
The acute and chronic effects of vitamin A toxicity are well documented
in the literature. Emerging evidence suggests that sub toxicity without
clinical signs of toxicity may be a growing concern, because intake from
preformed sources of vitamin A often exceeds the recommended dietary allowances
(RDA) for adults, especially in developed countries. Fatigue, headache,
malaise, lack of muscular coordination may be symptoms of this sub toxicity
[15].
In a trial in Zanzibari, vitamin A significantly decreased
erythropoietin concentration [16].
Vitamin A has a long biologic half-life and bioaccumulates. The
combination of relatively rapid absorption with a low clearance can produce
acute toxicity within hours after a sufficiently high dose and chronic toxicity
after prolonged intake of substantially smaller doses. This may lead to anemia
and thrombocytopenia [17].
Even low intakes of vitamin A in early pregnancy are associated with
congenital malformations. Vitamins released from the malaria parasites enter
the fetus and cause preterm birth and/or low birth weight [18].
The interaction of taurine and vitamin A however seems to have positive
effects. Symptoms of vitamin A toxicity in rats including loss of body weight,
hepatotoxicity and nephrotoxicity were significantly reduced when the rats were
fed the diet with the supplement of taurine in rats [19].
Helminths and vitamin A
Helminths are among the most common chronic infections in the tropics
and Plasmodium infections the most deadly. These two groups of parasites have
similar geographical distribution and co-infection is commonplace. It has
increasingly been speculated that helminth infections may alter susceptibility
to clinical malaria [20].
Co-infection with helminths seems to protect against severe malaria.
This may be tentatively explained on the following hypothesis. The parasitic
worm Onchocerciasis volvulus,
like Plasmodium falciparum,
selectively absorbs and concentrates vitamin A, such that the concentration in O. volvulus is about eight
times higher than that in the surrounding tissues of the host. O. volvulus
reduces the availability of vitamin A for the malaria parasite in the early
sporozoite or blood stage of the lifecycle, which starves and weakens the
parasite, perhaps reducing the number of parasites reaching the liver and
thereby lessening symptom severity [21,22].
A study was undertaken in Turkey to study the influence of liver
parasites in cattle on the vitamin A content. Naturally infected cattle with D. dendriticum, F. hepatica and hydatid cyst showed lower vitamin A levels. This
study indicated that serum levels of vitamin A and β-carotene decline was
present in cattle with liver parasite infection [23].
A striking finding is the relative freedom from malaria in children of
Anjouan but not of Grande Comore, two neighboring islands of the Comorro group
in the Indian Ocean. Compared with those of Grande Comore, Anjouan children
were heavily infested with Ascaris lumbricoides [24,25].
The vitamin A business
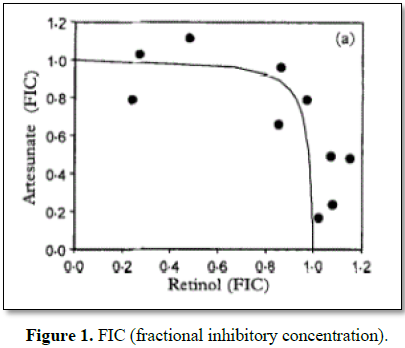
The antimalarial effect of vitamin A (retinol) is also complicated by
the fact that it strongly antagonizes artemisinin and its derivatives.
Plasmodium uses vitamin E, C and A to avoid oxidative stress. It is disturbing
to notice that this effect is known since 20 years but is not taken into
consideration in the prescription of ACTs [26] (Figure 1).
Even worse, vitamin A is proposed as an adjuvant in vaccines [27].
It was also found that too much vitamin A shuts down the body’s trained
immunity, opening the door to infections to which we would otherwise be immune
[28].
Vaccines are developed against helminthic diseases, bluntly ignoring or
without considering the detrimental effect on malaria [29].
The obsession of inadequate levels in the human body goes so far as to
declare that breast milk does not contain enough vitamin A and that supplements
should be administered to lactating mothers. A study in Brazil found that only
38% 0f lactating women presented enough vitamin A concentrations in milk for
the infants. They do not consider the hypothesis that human milk is poor in
vitamin A to protect the infant against diseases, including malaria [30].
But vitamin sales to Africa are big business. A senior health adviser to
the US Agency for International Development (USAID) announced their plan to
supplement basic food products with vitamin A which will save millions of
children in Third World countries from death and diseases [31].
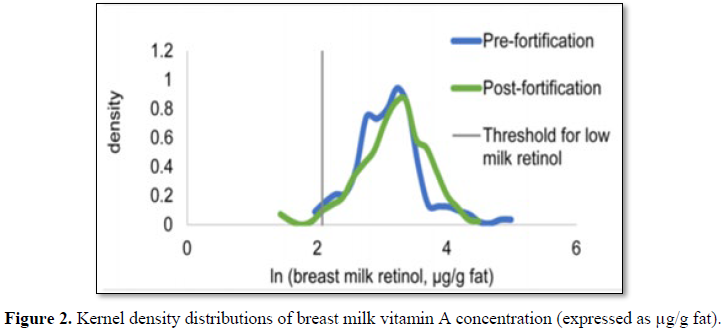
In Cameroon cooking oil is supplemented with vitamin A in a mandatory
national program. An assessment was made of the impact on some 300 people. No
significant difference was found on inflammation, malaria prevalence and
vitamin A content in breast milk. This may indicate that breast milk
autoregulates (homeostasis) its vitamin A content to avoid high concentrations [32]
(Figure 2).
Many Americans and Europeans get a lot of preformed vitamin A in their
diet and Norway and Germany have cautioned that any additional exposure could
increase the number of people at risk of hypervitaminosis A or excessive
vitamin A.
Artemisias are poor in vitamin A
The strong therapeutic and prophylactic properties of Artemisia annua
and Artemisia afra are far from being understood. A piece of the puzzle
could be that in all tissues (leaves, roots, stems, inflorescence) of the sun
dried plant vitamin A concentrations only are marginal: <0.3 µg/100 g.
Extremely low vitamin A contents were also found in 5 Artemisia species in
Turkey. This is in sharp contrast with the vitamin A content in dry green tea (Camellia
sinensis) leaves: 3.3 mg/100 g or 10000 times more. And this could
eventually explain the total absence of antimalarial properties of green tea. Moringa
oleifera is also very rich vitamin A, higher than in carrots and has no
known antimalarial properties.
It may also explain why Artemisia
vulgaris has no antimalarial properties. Vitamin A is found in mugwort at such high
concentrations that it is used for vision health, a well-known therapeutic
property of vitamin A. In Artemisia dracunculus the concentration is 100
µg/100 g or 1000 times more than in Artemisia annua [33-36].
1. Binka
FN, Ross DA, Morris SS, Kirkwood BR, Arthur P, et al. (1995) Vitamin A
supplementation and childhood malaria in northern Ghana. Am J Clin Nutr 61:
853-859.
2. Zekiba
T, Zongo Sorgho I, Rouamba N (2009) Impact of zinc and vitamin A
supplementation on malaria among children under 5 year in Burkina Faso.
MIM16690268 MIM Conference, Nairobi.
3. Shankar
HA (1995) Vitamin A and malaria. Am J Clin Nutr 62: 842.
4. Serghides
L, Kain KC (2002) Mechanism of protection induced by vitamin A in falciparum
malaria. Lancet 359: 1404-1406.
5. Yakoob
MY, Qadir M, Hany OE (2018) Vitamin A supplementation for prevention and
treatment of malaria during pregnancy and childhood: A systematic review and
meta-analysis. J Epidemiol Glob Health 8: 20-28.
6. Benzecry
SG, Alexandre MA Vítor-Silva S, Salinas JL, de Melo GC, et al. (2016)
Micronutrient deficiencies and Plasmodium
vivax malaria among children in the Brazilian Amazon. PLoS One 11:
e0151019.
7. Hautvast
JL, Tolboom JJ, West CE, Kafwembe EM, Sauerwein RW (1998) Malaria is associated
with reduced serum retinol levels in rural Zambian children. Int J Vitamin Nutr
Res 68: 384-388.
8. Mizuno
Y, Kawazu SI, Kano S (2003) In vitro uptake of vitamin A by Plasmodium falciparum. Ann Trop Med
Parasitol 97: 237-243.
9. Sirsjo
A, Gidlo AC, Olsson A (2000) Retinoic acid inhibits nitric oxide synthase-2
expression through the retinoic acid receptor-alpha. Biochem Biophys Res Commun
270: 846-851.
10. Worm
M, Krah JM, Manz RA, Henz BM (1998) Retinoic acid inhibits CD40 1 interleukin-4
mediated IgE production in vitro.
Blood 92: 17-23.
11. Bando
N, Yamanishi R, Terao J (2003) Inhibition of immunoglobulin E production in
allergic model mice by supplementation with vitamin E and beta-carotene. Biosci
Biotechnol Biochem 67: 2176-2182.
12. Mawson
AR (2013) The pathogenesis of malaria: A new perspective. Pathog Global Health
107: 122-129.
13. Hoglen
NC, Abril EA, Sauer JM, Earnest DL, McCuskey RS, et al. (1997) Modulation of
Kuppfer cell and peripheral monocyte activity by in vivo treatment of rats with all-trans-retinol. Liver 17:
157-165.
14. Ohata
M, Yamauchi M (2000) RAR and RXR expression by Kuppfer cells. Exp Mol Pathol
68: 13-20.
15. Penniston
KL, Tanumihardjo SA (2006) The acute and chronic toxic effects of vitamin A. Am
J Clin Nutr 83: 191-201.
16. Cusick
SE, Tielsch JM, Ramsan M, Jape JK, Sazawal S (2005) Short-term effects of
vitamin A and antimalarial treatment on erythropoiesis in severely anemic
Zanzibari preschool children. Am J Clin Nutr 82: 406-412.
17. Perrotta
S, Nobili B, Ragione FD, Rossi F, Criscuolo M, et al. (2002) Infant
hypervitaminosis A causes severe anemia and thrombocytopenia: Evidence of a
retinol-dependent bone marrow cell growth inhibition. Blood 99: 2017-2022.
18. Allen
LH, Haskell M (2002) Estimating the potential for vitamin A toxicity in women
and young children. J Nutr 132: 2907S-2919S.
19. Yeha
YH, Leeb YT, Hung-Sheng (2008) Effect of taurine on toxicity of vitamin A. Food
Chem 106: 260-268.
20. Tabitha
WM, Bethony J, Brooker S (2006) Malaria and helminth interactions in humans: An
epidemiological view point. Ann Trop Med Parasitol 100: 551-570.
21. Hartgers
FC, Yazdanbakhsh M (2006) Co-infection of helminths and malaria: Modulation of
the immune responses to malaria. Parasite Immunol 28: 497-506.
22. Sturchler
D, Wyss F, Hanck A (1981) Retinol, onchocerciasis and Onchocerca volvulus. Trans R Soc Trop Med Hyg 75: 617.
23. Jalilzadeh-Amin
G, Esmaeilnejad B, Farhang-Pajuh F (2017) Study on the relationship between
liver parasitic infections and serum vitamin A and β-carotene status in cattle.
Turkiye Parazitol Derg 41: 198-203.
24. Murray
MJ, Murray AB, Murray MB, Murray CJ (1977) Parotid enlargement, forehead edema
and suppression of malaria as nutritional consequences of ascariasis. Am J Clin
Nutr 30: 2117-2121.
25. Mwatha
JK, Jones FM, Mohamed G (2003) Associations between anti-Schistosoma mansoni and anti-Plasmodium
falciparum antibody responses and hepatosplenomegaly, in Kenyan
schoolchildren. J Infect Dis 187: 1337-1441.
26. Skinner-Adams
T, Barrett H, Davis TM (1999) Heterogeneous activity in vitro of vitamin A (retinol) in combination with novel and
established antimalarial drugs. Trans R Soc Trop Med Hyg 93: 550-551.
27. Patel
Skalkotkar A (2016) Vitamin A or E and a catechin synergize as vaccine adjuvant
to enhance immune responses in mice by induction of early interleukin-15 but
not interleukin-1β responses. Immunology 148: 352-362.
28. Arts
RJ, Blok BA, van Crevel R, Aaby P, Benn CS, et al. (2015) Vitamin A induces
inhibitory histone methylation modifications and down-regulates trained
immunity in human monocytes. J Leukoc Biol 98: 129-136.
29. Afzal
A, Bilal A (2011) Schistosomiasis vaccines. Hum Vaccin 7: 1192-1197.
30. Souza
G, Ramalho A (2012) Concentraçao de vitamina A no leite humano maduro. J
Pediatr (Rio J) 88: 496-502.
31. Barber
B (1998) Vitamin A: Lifesaver for the Third World. AID, private sector to bring
it Washington Times. A11.
32. Engle-Stone
R, Nankap M (2017) Vitamin A status of women and children in Yaoundé and
Douala, Cameroon, is unchanged one year after initiation of a National Vitamin
A Oil Fortification Program. Nutrients 9: 522.
33. Gayathri
P, Anitha T, Thomas L (2017) Comparison of biochemical, mineral and nutritive analysis
of Camellia sinensis L. (Green Tea)
with normal tea dust. Int J Adv Sci Res 2: 5-8.
34. Kurşat
M, Yılmaz O, Emre I, Civelek Ş, Gökçe Z (2015) Some biological contents and
radical scavenging activities of five Artemisia L. species growing in Turkey. J
Drug Metab Toxicol 5: 172.
35. Mann
C (2016) Der Gewöhnliche Beifuss (Artemisia
vulgaris) – eine Pflanze im Wandel der Zeit. Swiss J Integr Med.
36. Brisibe
EA, Ferreira JFS (2009) Nutritional characterization and antioxidant capacity
of different tissues of Artemisia annua
L. Food Chem 115: 1240-1246.