696
Views & Citations10
Likes & Shares
A novel series of benzofuran derivatives were synthesized via treatment
of 3-(5-bromobenzofuran-2-yl)-3-oxopropanenitrile 1 with diazonium salts of heterocyclic amines 2a-e and with aryl diazonium chlorides to give the new 3a-e and 5a,b derivatives, respectively . In addition, compound 1 was
reacted with hydrazonoyl halides 6a-c
to give pyrazoles 8a-c. On the other
hand, 3-(benzofuran-2-yl)-3-oxopropanenitrile 9 was reacted with phenyl
isothiocyanate and each of ethyl chloroacetate, chloroacetone and
chloroacetonitrile to give compounds 10-12,
respectively. The structures of the newly synthesized compounds were elucidated
based on their spectral data and elemental analysis, whenever possible.
Keywords: Benzofuran
derivatives, 3-oxopropanenitrile, Aryldiazonium chloride, Hydrazonoyl halides
INTRODUCTION
EXPERIMENTAL
All melting points were
determined on an electrothermal apparatus and were uncorrected. IR spectra were
recorded (KBr discs) on a Shimadzu FT-IR 8201 PC spectrophotometer. 1H
and 13C NMR spectra were recorded in CDCl3 and (CD3)2SO
solutions on a Varian Gemini 300 MHz and JNM-LA 400 FT-NMR system spectrometer
and chemical shifts are expressed in ppm units using TMS as an internal
reference. Mass spectra were recorded on a GC-MS QP1000 EX Shimadzu. Elemental
analyses were carried out at the Microanalytical Center of Cairo University.
Synthesis of 3a-e, 5a,b, 11 and 12a-c
A solution of the
appropriate diazonium salt of amines (5 mmol) was added to a mixture of
3-(5-bromobenzofuran-2-yl)-3-oxopropanenitrile or 4-(5-bromobenzofuran-2-yl)-thiazole-2-amine
10 (5 mmol) and sodium acetate (0.65 g, 5 mmol) in ethanol (30 mL) at
0-5°C while stirring. The resulting solid which formed after 2 h was collected,
washed with water and recrystallized from a proper solvent to give 3a-e,
5a,b, 11 and 12a-c, respectively.
(E)-2-(2-(5-phenyl-1H-pyrazol-5-yl)hydrazono)-3-(5-bromobenzofuran-2-yl)-3-oxopropanenitrile
3a
Brown crystals from dioxane,
yield (75%), mp: 256-259°C; IR (KBr): 3334, 3166 (2NH), 3065 (CH, aromatic),
2224 (CN), 1640 (C=O); 1H NMR δ=6.46 (s, 1H, pyrazole), 7.21-7.79 (m, 9H,
ArH's); 8.81,9.24 (s, 2H, 2NH). Anal. Calcd. for C20H12BrN5O2
(434.25): C, 55.32; H, 2.79; Br, 18.40; N, 16.13. Found: C, 55.36; H, 2.75; Br,
18.44; N, 16.17.
(E)-2-(2-(4-phenyl-1H-pyrazol-5-yl)hydrazono)-3-(5-bromobenzofuran-2-yl)-3-oxopropanenitrile
3b
Yellow crystals from AcOH,
yield (75%), mp: 260-263°C; IR (KBr): 3051 (CH, aromatic), 2230 (CN), 1658
(C=O); 1H NMR δ=6.43 (s, 1H, pyrazole), 7.21-7.72 (m, 9H, ArH's); 8.80, 9.0 (s,
2H, 2NH). Anal. Calcd. For C20H12BrN5O2
(434.25): C, 55.32; H, 2.79; Br, 18.40; N, 16.13. Found: C, 55.36; H, 2.75; Br,
18.44; N, 16.17.
(E)-2-(2-(4-cyano-1H-pyrazol-5-yl)hydrazono)-3-(5-bromobenzofuran-2-yl)-3-oxopropanenitrile
3c
Brown crystals from dioxane,
yield (84%), mp: >300°C; IR (KBr): 3340 (NH), 2220 (CN); 1H NMR
δ=7.20-7.71 (m, 7H, ArH's and 2NH). Anal. Calcd. for C15H7BrN6O2
(383.16): C, 47.02; H, 1.84; Br, 20.85; N, 21.93. Found: C, 47.06; H, 1.80; Br,
20.81; N, 21.97.
(E)-2-(2-(1H-1,2,3-triazol-1-yl)hydrazono)-3-(5-bromobenzofuran-2-yl)-3-oxopropanenitrile
3d
Brown crystals from AcOH,
yield (84%), mp: 284-86°C; IR (KBr): 3300 (NH), 2215 (CN), 1675 (CO); 1H NMR
δ=7.10-7.95 (m, 8H, ArH’s+NH). Anal. Calcd. for C13H7BrN6O2
(359.14): C, 43.48; H, 1.96; Br, 22.25; N, 23.40. Found: C, 43.44; H, 1.92; Br,
22.21; N, 23.44.
(E)-2-(2-(1H-benzo[d]imidazol-2-yl)hydrazono)-3-(5-bromobenzofuran-2-yl)-3-oxopropanenitrile
3e
Brown powder from AcOH,
yield (72%), mp: >300°C; IR (KBr): 3320, 3183 (2NH), 2224 (CN), 1668 (CO);
1H NMR δ=7.07-8.11 (m, 10H, ArH's). Anal. Calcd. For C18H10BrN5O2
(408.21): C, 52.96; H, 2.47; Br, 19.57; N, 17.16. Found: C, 52.92; H, 2.43; Br,
19.53; N, 17.14.
2-(benzofuran-2-yl)-2-oxo-N'-phenylacetohydrazonoyl
cyanide 5a
Brown powder from AcOH,
yield 85%, mp: 210-12°C; IR (KBr): 3190 (NH), 3076 (CH, aromatic), 2223 (CN),
1710 (CO): 1H NMR δ=7.20-7.96 (m, 9H, ArH's),12.46 (s,1H, NH). Anal. Calcd. for
C17H10BrN3O2 (368.18): C, 55.46; H,
2.74; Br, 21.70; N, 11.41.
2-(benzofuran-2-yl)-2-oxo-N'-(p-tolyl)phenylacetohydrazonoyl
cyanide 5b
Brown powder from EtOH,
yield (83%), mp: 195-97°C; IR (KBr): 3205 (NH), 3040 (CH, aromatic), 2209 (CN),
1634 (CO); 1H NMR δ=2.40 (s, 3H, CH3) 7.23-8.22 (m, 8H, ArH's),
15.44 (s, 1H, NH). Anal. Calcd. for C18H12BrN3O2
(382.21): C, 56.56; H, 3.16; Br, 20.91; N, 10.99. Found: C, 56.52; H, 3.12; Br,
20.95; N, 10.95.
Synthesis of 3-substituted
5-(benzofuran-2-yl)4-cyano-1-phenyl-1H-pyrazole 8a-c
Compound 1 (5 mmol)
was added to a stirred ethanolic sodium ethoxide solution (0.12 g sodium metal
in absolute ethanol 20 mL). After 20 min., the appropriate hydrazonoyl halide 6a-c
(5 mmol) was added and the reaction mixture was stirred for 4 h. The resulting
solid was collected by filtration, dried and recrystallized from a proper
solvent to give 8a-c, respectively.
Ethyl-5-(bromobenzofuran-2-yl)-4-cyano-1-phenyl-1H-pyrazole-3-carboxylate
8a
Yellow crystals from
ethanol. yield (83%), mp.: 193°C. FT-IR (KBr, cm-1): 3066v
(CH-aroma.), 2995, 2915v (CH-aliph), 2232v (CN), 1727v (CO), 1585v (C=C). 1H
NMR (300 MHz, DMSO-d6, δ, ppm): 1.20 (t, 3H, J=7 Hz, CH2CH3), 4.33 (q, 2H, J=7
Hz, CH2CH3), 6.90 (s, 1H, CH-furan), 7.39-7.60 (m, 8H,
ArH's). Anal. Calcd. for C21H14BrN3O3
(436.26): C, 57.82; H, 3.23; Br, 18.32; N, 9.63. Found: C, 57.86; H, 3.27; Br,
18.36; N, 9.67.
3-Acetyl-5-(bromobenzofuran-2-yl)-1-phenyl-1H-pyrazole-4-carbonitrile
8b
Yellow crystals from acetic
acid; yield (79%), m.p. 244-246°C. FT-IR (KBr, cm-1): 3060 v
(CH-arom.), 2229 v (CN), 1697 v (CO), 1600 v (C=N). 1H NMR (300 MHz, DMSO-d6,
δ, ppm): 2.60 (s, 3H, CH3), 7.00 (s, 1H, CH-furan), 7.20-7.65 (m, 8H, ArH's).
Anal. Calcd. for C20H12BrN3O (390.23): C,
61.56; H, 3.10; Br, 20.48; N, 10.77. Found: C, 61.52; H, 3.14; Br, 20.44; N,
10.73.
3-(Bromobenzofuran-2-yl-carbonyl)-5-(benzofuran-2-yl)-1-phenyl-1H-pyrazole-4-carbonitrile
8c
Red crystals from ethanol.
Yield: 80%, m.p. 227-230°C. 1H NMR (300 MHz, DMSO-d6, δ, ppm): 7.23-7.55 (m,
14H, ArH's). Anal. Calcd. for C27H14BrN3O3
(508.32): C, 63.80; H, 2.78; Br, 15.72; N, 8.27. Found: C, 63.84; H, 2.74; Br,
15.68; N, 8.31.
Synthesis of 10, 11a and 11b
A mixture of compound 9
(10 mmol), phenyl isothiocyanate (10 mmol) and potassium hydroxide (10 mmol) in
N, N-dimethylformamide (10 mL) was stirred for 2 h at room temperature. The
appropriate of ethyl chloroacetate, chloro acetyl chloride, chloroacetone or
chloroacetonitrile (10 mmol) was added while stirring. Stirring was continued
for 2 h. The resulting solid was collected and crystallized from a proper
solvent affording 10, 11a and 11b, respectively.
3-(benzofuran-2-yl)-3-oxo-2-(4-oxo-3-phenylthiazolidin-2-yl)propanenitrile
10
Brown crystals from ethanol.
Yield: 81% mp: 283-285°C; FT-IR (KBr, cm-1): 3062 v (CH-aroma.),
2931, 2873 v (CH-aliph.), 2182 v (CN), 1664 v (CO), 1600 v (C=N). 1H NMR (300
MHz, DMSO-d6, δ, ppm): 4.10 (s, 2H, CH2), 6.90 (s, 1H, CH-furan) and
7.41-7.63 (m, 9H, ArH's). Anal. Calcd. for C20H12N2O3S
(360.39): C, 66.65; H, 3.36; N, 7.77. Found: C, 66.61; H, 3.32; N, 7.72.
3-Amino-4-(benzofuran-2-yl-carbonyl)-5-phenylamino-thiophen-2-yl)ethanone
11
Brown crystals from ethanol.
Yield 75% m.p. 280°C; FT-IR (KBr, cm-1): 3240 v (NH), 3031 v
(CH-arom.), 1670 (C=O), 1606 v (C=N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 4.20
(s, 2H, CH2), 2.40 (s, 3H, CH3), 4.20 (s, 1H, NH), 4.71
(s, 2H, NH2), 6.90 (s, 1H, CH-furan), 7.41-7.63 (m, 9H, ArH's).
Anal. Calcd. for C21H16N2O3S (376.43):
C, 67.00; H, 4.28; N, 7.44; S, 8.52. Found: C, 67.13; H, 4.15; N, 7.37; S,
8.67.
2-(benzofuran-2-yl-carbonyl)-3-phenylamino-3(cyanomethylthio)acrylonitrile
12
Brown crystals from ethanol.
Yield 75% mp. 160°C; FT-IR (KBr, cm-1): 3128 v (NH), 3058 v (CH-aroma.),
2175 v (CN) 1H NMR (300 MHz, DMSO-d6, δ, ppm): 4.20 (s, 2H, CH2), 4.20 (s, 1H,
NH), 6.81 (s, 1H, CH-furan) , 7.40-7.59 (m, 9H, ArH's). Anal. Calcd. for C20H13N3O2S
(359.4): C, 66.84; H, 3.65; N, 11.69. Found: C, 66.70; H, 3.55; N, 11.59.
RESULTS AND DISCUSSION
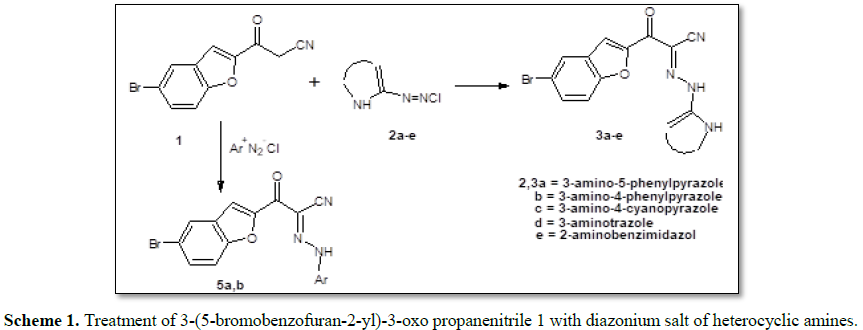
Treatment of
3-(5-bromobenzofuran-2-yl)-3-oxo propanenitrile 1 with diazonium salt of
heterocyclic amines 2a-e in ethanol containing sodium acetate solution
under stirring afforded 3a-e, respectively (Scheme 1). The
structures of the products were confirmed on the basis of elemental analysis,
spectral data. Thus, 1HNMR of 3a revealed signals at δ=6.48 (s, 1H,
pyrazole), 7.21-7.79 (m, 9H, ArH's); 8.81, 9.24 (s, 2H, 2NH).
In a similar manner,
compound 1 reacted with each of benzenediazonium chloride and 4-methyl
benzenediazonium chloride in ethanol containing sodium acetate to afford 5a
and 5b, respectively (Scheme 1). The structure of 5a,b
were confirmed based on elemental analysis and spectral data.
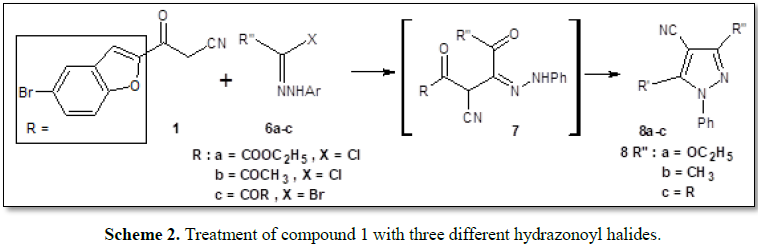
Furthermore, treatment of compound 1 with three
different hydrazonoyl halides [9-12] 6a-c gave products generally
assigned as 3-substituted
5-(5-bromobenzofuran-2-yl)-4-cyano-1-phenyl-1H-pyrazole derivatives 8a-c
on the basis of their analytical and spectral data (Scheme 2).
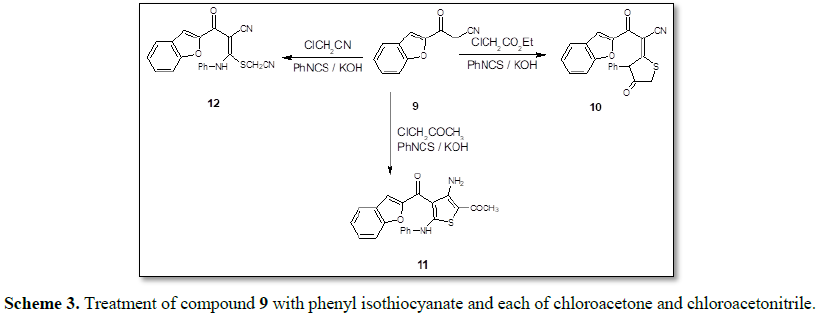
On the other hand,
3-(benzofuran-2-yl)-3-oxopropanenitrile [13,14] 9 reacted with phenyl
isothiocyanate and ethyl chloroacetate in N,N-dimethylformamide under stirring
at room temperature affording
3-(benzofuran-2-yl)-3-oxo-2(4-oxo-3-phenylthiazolidin-2-ylidene)propanenitrile 10
(Scheme 3). The IR spectra of 10 displayed an absorption band at
2931, 2873 v (CH-aliph.), 2171 v (CN) and 1660 v (C=O). It’s 1H NMR
in (DMSO-d6) revealed signals at δ 4.10 (s, 2H, CH2),
6.90 (s, 1H, CH-furan), 7.41-7.63 (m, 9H, ArH's). In a similar manner, compound
9 reacted with phenyl isothiocyanate and each of chloroacetone and
chloroacetonitrile yielding 2-(benzofuran-2-yl-carbonyl)-3-phenylamino-3-(acetylmethylthio)propanenitrile
11 and 2-(benzofuran-2-yl-carbonyl)-3-phenyl amino-3(cyanomethylthio)
propanenitrile 12, respectively (Scheme 3).
1. Abdel‐Aziem
A (2015) An efficient and simple synthesis of 2,3-dihydro-1,3,4-thiadiazoles,
pyazoles and coumarins containing benzofuran moiety using both conventional and
grinding methods. Int J Pharm Sci 7: 32.
2. Abdel‐Aziem
A, AbdelhamidA O (2013) One pot synthesis of pyridine, thiazolidine, pyrazole
and 2, 3-dihydro-1, 3, 4-thiadiazole derivatives under solvent-free condition.
Int J Adv Res 9: 717-728.
3. Halawa
AH (2014) Synthesis, reactions and biological evaluation of some novel
5-bromobenzofuran-based heterocycles. World J Org Chem 2: 9-17.
4. Santana
L, Teijeira M, Uriarte E, Teran C, Linares B, et al. (1998) AM1 theoretical
study, synthesis and biological evaluation of some benzofuran analogues of
anti-inflammatory aryl alkanoic acids. Eur J Pharm Sci 7: 161-166.
5. Findlay
JA, Buthelezi S, Li G, Seveck M (1997). Insect toxin from an endophytic fungus
from wintergreen. J Nat Prod 60: 1214-1215.
6. Dawood
KM, Abdel-Gawad H, Rageb EA, Ellithey M, Mohamad HA (2006) Synthesis,
anti-convulsant and anti-inflammatory evaluation of some new benzotriazole and
benzofuran based heterocycles. Bioorg Med Chem 14: 3672-3680.
7. Rida
SM, EI-Hawash SAM, Fahmy HTY, Hazzaa AA, EIMeligy MMM (2006) Synthesis of novel
benzofuran and related benzimidazole derivative for evaluation of in vitro anti-HIV-1, anticancer and
antimicrobial activites. Arch Pham Res 29: 826-833.
8. Raj
PA, Suddendra G, Shakeeland AS, Girish M (2012) Synthesis of new benzofuran
derivatives and their in vitro
evaluation for antimicrobial activity. Inter J Drug Formulation Res 3: 135-147.
9. Eweiss
NE, Osman A (1979) Synthesis of heterocycles. One step synthesis of acetyl
thiadiazolines. Tetrahedron Lett 13: 1169-1170.
10. Shawali
AS, Abdelhamid AO (1976) Reaction of dimethylphenacylsulfonium bromide with
N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull
Soc Chim Jpn 49: 321-324.
11. Shawali
AS, Osman A (1971) Synthesis and reactions of phenylcarbamoyl-arylhydrazidic
chlorides. Tetrahedron 27: 2517-2528.
12. Abdelhamid
AO, Attaby FA, Zaki MY (1990) Reactions with 2-(thiocyanatoacetyl) and
2-(selenacyanoacetyl)-2-benzofuran. Synthesis of some new thiadiazoline,
selenadiazoline, thiadiazolo[2,3-b] quinazopline and arylazothiazole
derivatives. Phosphorous, Sulfur, Silicon and Related Element 53: 403-410.
13. Yilmaz
M, Uzunalioglu N, Pekel AT (2005) Manganese (III) acetate based oxidative
cyclizations of 3-oxopropanenitriles with conjugated alkenes and synthesis of
4,5-dihydrofuran-3-carbonitriles containing heterocycles. Tetrahedron 61: 8860.
14. Yılmaz
EVB, Yılmaz M, Oktemer A (2011) Radical cyclizations of conjugated esters and
amides with 3-oxopropanenitriles mediated by manganese (III) acetate. Arkivoc
2: 363-376.