661
Views & Citations10
Likes & Shares
A new chromophore, 2-acetylthiophene isonicotinoylhydrazone (ATINH) has
been synthesized and used for non-extractive spectrophotometric determination
of cobalt (II). The metal ion reacts with the reagent in aqueous dimethyl
formamide (DMF) in wide pH range forming yellowish coloured 1:2 (M:L) soluble
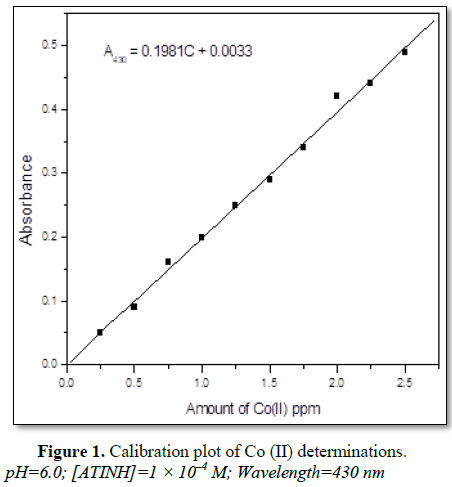
complex with lmax at 430 nm. Beer’s
law obeys in the range 5.893-58.93 μg mL-1 of Co (II). The molar
absorptivity, Sandell’s sensitivity, detection limit, determination limit and
relative standard deviation are calculated as 5.0 × 104 L mol-1cm-1,
0.001178 μg cm-2, 0.04 μg mL-1, 0.012 μg mL-1
and 0.998%, respectively. Various diverse ions which generally associated with
the determination of Cobalt (II) are also studies. The proposed method is
applied for the determination of cobalt(II) in, environmental and pharmaceutical
samples and the results were compared with standard dithizone method are quite
encouraging and have high precision and accuracy.
Keywords: 2-Acetylthiophene
isonicotinoylhydrazone, Cobalt, Spectrophotometry, Environmental and
pharmaceutical samples
INTRODUCTION
Cobalt is naturally occurring element found in rocks,
soil, water, plants and animals. Cobalt is used to produce alloys used in the
manufacture of aircraft engines, magnets, grinding and cutting tools, special
cobalt-chromium-molybdenum alloys are used for prosthetic parts such as hip and
knee replacements [1]. Iron-cobalt alloys are used for dental prosthetics [2].
Cobalt compounds are also used to color glass, ceramics and paints products [3]
and used as a drier for porcelain enamel and paints, fertilizers, feeds and
disinfectants. They are important building components in biological systems
[4].
Radioactive cobalt is used for commercial and medical
purposes. 60Co (read as cobalt sixty) is used for sterilizing medical equipment
and consumer products, radiation therapy for treating cancer patients, manufacturing
plastics an irradiating food. 57Co is used in medical and scientific research.
Review of literature reveals that a large number of
chromogenic reagents for the spectrophotometric determination of cobalt [5-11]
were recently proposed are less sensitive and less selective. We are now
proposing simple, sensitive and selective, direct and non-extractive
spectrophotometric methods for the determination of cobalt (II) in various
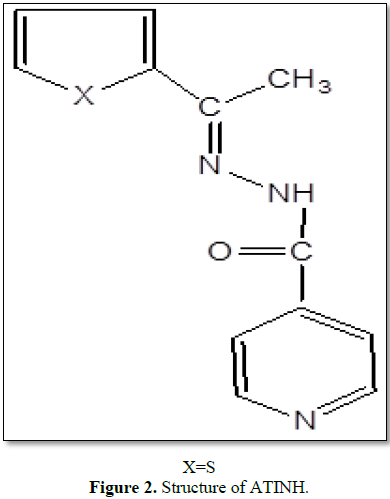
complex materials using 2-acetylthiophene isonicotinoylhydrazone (ATINH) as
chromogenic agent.
EXPERIMENTAL
Chemicals and reagents
Preparation of ATINH: In a 250 ml Erlenmeyer
flask, a hot methonolic solution (10 ml) of 2-aecetylthiophene (0.05 mol) and
isoniazid (0.05 mol), dissolved in 10 ml of hot distilled water were taken.
Suitable quantity (~2 ml) of dilute hydrochloric acid was added to the reaction
mixture and refluxed for 4 h. On cooling the reaction mixture, pale yellow
colored product (ATINH) was separated out. It was collected by filtration and
washed several times with hot water and 50% cold methanol. This compound was
re-crystallized from ethanol and dried in vacuuo; Yield 6.6 g; M.P.196°C.
Characterization of ATINH: The compound was
characterized by IR and 1H-NMR, Mass and UV-Visible spectral data. Infrared
spectrum of ATINH shows bands at 3281(s), 3059(m), 2927(m), 1666(s), 1599(s),
1548(s), 1500(m), 1482(m), 1297(m) and 747(m) cm-1, respectively
corresponding to u(NH) secondary, u(C-H) aromatic stretch, u(C-H) aliphatic stretching, u(C=O) hydrazine, u(C=N) azomethine, u(C-C) aromatic ring, u(C-C) aromatic ring, u(C-C) aromatic ring, u(C-N) stretch, u(C-H) oop bend(thiophene) aromatic ring vibrations.
1H-NMR spectrum of ATINH (CDCl3+DMSO-d6)
showed signals at 2.4 (3H, s), 6.5-8.0 (7H, m) and 10.5 (1H, s) due to CH3 and
isonicotine+thiophene proton, -NH(imino) groups of hydrazine, respectively.
Mass spectrum of ATINH shows molecular ion peak at
m/z 246 corresponding to the molecular weight. Other peaks due to loss of
methyl radical and thiophene radical are also observed in mass spectrum.
Instrumentation
Shimadzu 160A UV-Visible spectrophotometer equipped
with 1.0 pm quartz cell and an Elico model LI-610 pH meter were used in the
present study (Figure 1).
Preparation of Cobalt (II) solution: Stock solution
of Co (II) (1 × 10–2 M) was prepared from CoCl2 6H2O
with doubly distilled water containing few drops of concentrated HCl and made
up to 100 ml. The resulting solution was standardized.
Determination of Cobalt (II) in various water, soil and pharmaceutical
samples: The present method is applied for the
determination of Co (II) in water samples, soil and pharmaceutical samples.
Water samples [12,13]
Each filtered (with whattman No. 40) water sample
(250 ml) was mixed with 10 ml of concentrated nitric acid in a 500 ml
distillation flask. The sample was digested in the presence of an excess
potassium permanganate solution according to the method recommended by Fifield
et al. [12]. The solution was cooled and neutralized with dilute NH4OH
solution. The digest was transferred into a 25 ml calibrated flask and diluted
up to the mark with deionized water. The results were given in Table 1.
Preparation of soil samples [14]
2 g weight of soil, 5-7 ml of concentrated H2SO4 and an excess of KMnO4 are mixed in a conical flask equipped with a reflux condenser. The crystals of KMnO4 are added slowly in small portions, while stirring. It is heated until vapors of SO3 are evolved. After cooling down, 10 ml of distilled water are added. The excess of KMnO4 and manganese oxides are eliminated by adding H2O2. Iron is isolated by precipitation as hydroxide. After filtration, the solution is transferred into 25 ml standard flask and the volume is brought to the mark with distilled water. Aliquots of this solution were taken for analysis by following procedure given above. The results were given in Table 1.
A known quantity of the pharmaceutical sample (Neurobian Forte/Basiton Forte, Cyanocobalamine - 15 mg of each sample) was taken in a beaker and dissolved in minimum volume of alcohol. Then added 3 mL of 0.01 M nitric acid and evaporated to dryness. The dried mass was again dissolved in alcohol. This was filtered through Whatman filter paper and the filtrate was diluted to 100 mL with distilled water. The lower concentrations were prepared by the appropriate dilution of the stock solution. The results were given in Table 2.
Recommended procedure [16,17]
An aliquot of the solution containing cobalt in
Beer’s law validity range, 10 ml of NaOAc-AcOH buffer solution (pH 6.0) and 1.0
ml of 0.01 M ATINH were mixed in a 25 ml volumetric flask and resulting
solution was diluted to the mark with distilled water. The absorbance of this
solution was measured at 430 nm against reagent blank. The measured absorbance
is used to compute the amount of cobalt present in the samples using
pre-determined calibration plot.
Dithizone method [18]
As stated in the recommended procedure in a 25 ml volumetric flask 10 ml of buffer (pH 6.0), aliquots of cobalt in beer’s law validity range, and instead of reagent ATINH, 1.0 ml dithizone solution is added and the resulting solution was made up to the mark with distilled water. The absorption is measured at 430 nm against reagent blank.
Composition and stability constant of the complex
The composition of the complex was determined by
Job’s continuous variation and molar ratio methods. The stability constants of
the complexes were calculated by using the data obtained from Job’s plot.
Job’s continuous variation method
In a set of (nine) 25 ml volumetric flasks, 10 ml of
buffer solution and 1.0 ml of DMF were added to each flask. Equimolar solutions
of metal ion and reagent in different volume proportions (keeping the volume at
10 ml) were added to each flask. The contents in each flask were made up to the
mark with distilled water. The absorbance of the complex in each flask was
measured at 430 nm wavelength against a reagent blank. A plot between mole
fraction of reagent and the absorbance was made from which the composition of
the complex was computed.
Molar ratio method
In a set of (ten) 25 ml calibrated flasks, 10 ml of
buffer solution, 1.0 ml of DMF, constant amount of metal ion and varying
aliquots of the reagent solutions were added. The contents of each flask were
made upto the mark with distilled water. The absorbance of the colored complex
in each flask was measured at 430 nm against the reagent blank. From the plot
between the absorbance and mole of the reagent per mole of metal ion, the
composition of the complex was ascertained.
RESULTS AND DISCUSSION
The reagent ATINH may be easily prepared under reflux conditions. The reagent solutions (0.01 M) are found to be stable for 12 h. The bathochromic shift of absorption band 270-370 nm indicates that in solution are increasing the pH, the acid is neutralized and the C=S group enolised and dissociated [14]. In basic medium (above pH 7.3) the ligand presumably exists in enolic form and coordinates the divalent metal ion as mono anion to give neutral complexes. The structure of ATINH is given in Figure 2.
The Cobalt (II) reacts with ATINH in acidic pH to
give water soluble complexes. The color reactions are instantaneous at room
temperature. The change in the order of addition of metal ion, reagent (ATINH)
and buffer has no effect in the absorbance of complexes. The solubility of
reagents and their metal complexes are low in aqueous medium. But it was
observed that the reagents and their metal complexes were more easily soluble
in aqueous DMF. In order to arrive at the optimum percentage of DMF required retaining
them in solution different aliquots of DMF added to the sample solutions. An
analytical characteristic of the complex is summarized in Table 3. The
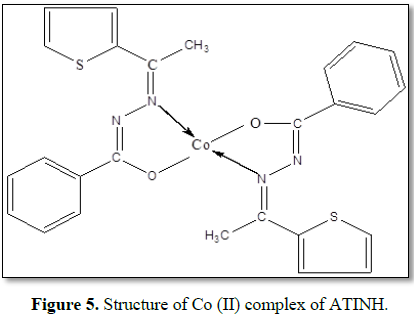
stoichiometry of the complex (M:L=1:2) was determined by Job’s continuous
variation method and molar ratio methods. Sodium acetate (0.2 M) - acetic acid
(0.2 M) buffer solution (pH 6.0 and T=300 k) and equimolar 4.8 × 10-5
M solutions of Co (II) or ATINH were used in this method. The stability
constant of the complex 5.75 × 1013 M is calculated using α (0.08)
and C (2.8 × 10-5 M) values obtained in Job’s method using the
formula:
β1:2 = 1-α / 4α3 C2
The structure of Co
(ATINH)2 complex is given in Figures 3-5.
Tolerance limits of foreign ions
The effect of foreign ions was studied with a view
to examine the applicability of the method in presence of foreign ions.
Interference of various ions was studied with 5.893 μg mL-1 of
cobalt by adopting the procedure. The tolerance limit value was taken as the
amount of foreign ion required to cause ± 2% error in the absorbance of the Co
(II)–ATINH complex (Table 4).
Applications
Cobalt was estimated in various water samples,
biological, soil and pharmaceutical samples by employing the present method.
The results are presented in Tables 1 and 2.
CONCLUSION
The study reveals that the Cobalt content can be
determined in ppm level in water, soil and pharmaceutical samples using present
method. All these findings cause great concern regarding public health
demanding an accurate determination of this metal ion at trace levels and the
present study may provide awareness among the public.
Acknowledgement
The authors are grateful to Department of Chemistry, Sri Krishnadevaraya University, Anantapur for allowing to carry out the present work. Authors also thank Dr. B.V. Subba Reddy and K. Venkateswarlu of IICT, Hyderabad for providing IR and NMR spectral data.
1. Michel
R, Nolte M, Reich M, Loer F (1991) Systemic effects of implanted prostheses
made of cobalt-chromium alloys. Arch Orthopedic Trauma Surg 110: 61-74.
2. Disegi
JA, Kennedy RL, Pillia R (1999) Cobalt-base alloys for biomedical applications.
ASTM Int Standards.
3. Adolfsson
MLC, Saloranta AK, Silander MK (1999) Colorant composition for paint products.
US Patent, Patent number: 5985987.
4. Hentze
MW, Kühn LC (1996) Molecular control of vertebrate iron metabolism: mRNA-based
regulatory circuits operated by iron, nitric oxide and oxidative stress. Proc
Natl Acad Sci U S A 93: 8175-8182.
5. Malik
AK, Kaul KN, Lark BS, Faubel W, Rao ALJ (2001) Spectrophotometric determination
of cobalt, nickel palladium, copper, ruthenium and molybdenum using sodium
isoamylxanthate in presence of surfactants. Turk J Chem 25: 99-105.
6. Reddy
BR, Radhika P, Kumar JR, Priya DN, Rajgopal K (2004) Extractive
spectrophotometric determination of cobalt(II) in synthetic and pharmaceutical
samples using cyanex. Anal Sci 20: 345-349.
7. Shar
GA, Soomro GA (2004) Spectrophotometric determination of cobalt(II), nickel(II)
and copper (II) with 1-(2-yridylazo)-2-naphthol in micellar medium. Nucleus 41:
77-82.
8. Veerachalee
N, Taweema P, Songsasen A (2007) Complexation and spectrophotometric
determination of cobalt(II) ion with 3-(2-thiazolylazo)-2,6-diaminopyridine.
Kasetsart J Nat Sci 41: 675-680.
9. Haoyi
Y, Guoxiu Z, Gaohua Y (2009) Determination of cobalt in terephthalic acid by
picramazochrom spectrophotometry. Chem Anal Meterage 1.
10. Guzar
SH, Jin QH (2008) Simple, selective and sensitive spectrophotometric method for
determination of trace amounts of nickel(II), copper (II), cobalt (II) and iron
(III) with a novel reagent 2-pyridine carboxaldehyde isonicotinyl hydrazine.
Chem Res Chin Univ 24: 143-147.
11. Prabhulkar
SG, Patil RM (2008) 2-Hydroxy-1-naphthalidine salicylohydrazone as an
analytical reagent for extractive spectrophotometric determination of a
biologically and industrially important metal Cobalt(II). Int J Chem Sci 6:
1480-1485.
12. Fifield
FW, Haines PJ (2000) Environmental analytical chemistry. Blackwell Science, p:
378.
13. Marczenko
Z (1976) Spectrophotometric determination of elements. Wiley: New York, pp:
241, 351, 602
14. Kamburova
M (1993) Spectrophotometric determination of mercury in soils with
triphenyltetrazolium chloride. Talanta 40: 719-723.
15. Devi
VSA, Reddy VK (2013) Spectrophotometric determination of iron(II) and
cobalt(II) by direct, derivative and simultaneous methods using 2-hydroxy-1-naphthaldehyde-p-hydroxybenzoichydrazone.
Int J Anal Chem 2013: 1-12.
16. Reddy
KH (1983) Ph.D. thesis. S.K. University.
17. Vogel
AI (1975) A Text Book of Quantitative Inorganic Analysis. 3rd Edn.
ELBS and Longman, p: 325.
18.
Snell FD, Snell CT (1949) Colorimetric
methods of analysis. 3rd Edn. p: 11, 92.